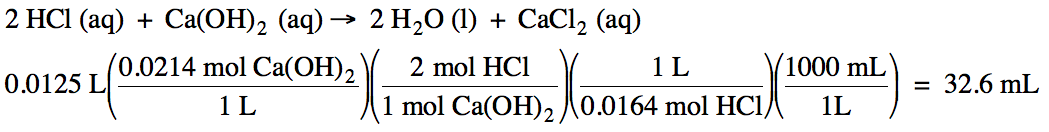Chapter 10: Chemical Tests and Chemical Reaction
Types
Section 10-1: Laboratory Tests to Identify Chemicals
Section 10-2: Diatomic Nonmetals
Section 10-3: Combustion of Organic Compounds
Section 10-4: Reactions Involving Metals or Metal Compounds
Section 10-5: Aqueous Ionic Compounds and Molarity of Ions
Section 10-6: Solubility Rules, Precipitation Reactions, and Net Ionic Equations
Section 10-7: Acid + Base Neutralization Reactions and Molecular Equations
Section 10-8: Experiment - Acid + Base Titration
Chapter 10 Practice Exercises and Review Quizzes
Section 10-1: Laboratory Tests to Identify Chemicals
The following lab tests have been
used to indicate the likely presence of the common chemicals listed:
|
Chemical |
Test |
|
carbon dioxide gas, CO2 (g) |
flaming wooden splint extinguished |
|
hydrogen gas, H2 (g) |
flaming wooden splint "pops" |
|
oxygen gas, O2 (g) |
glowing wooden splint relights |
|
significant concentration of aqueous
hydrogen ion, H+
(aq) |
solution is acidic, so: 1. low pH meter reading 2. litmus paper will be red 3. phenolphthalein indicator will be colorless |
|
significant
concentration of aqueous hydroxide ion,
OH- (aq) |
solution is basic, so: 1. high pH meter reading 2. litmus paper will be blue 3. phenolphthalein indicator will be pink |
Section 10-2: Diatomic Nonmetals
There are 7 nonmetals that exist in
covalently-bonded pairs at room temperature and pressure. Each of these is listed below along
with its state of matter at room temperature and pressure:
hydrogen gas, H2 (g)
nitrogen gas, N2 (g)
fluorine gas, F2 (g)
chlorine gas, Cl2 (g)
liquid bromine, Br2 (l)
solid iodine, I2 (s)
When the elements above are
reactants or products in a chemical reaction, they should be written as
diatomic.
Section 10-3: Combustion of Organic Compounds
The complete combustion or burning
of organic compounds with the condensed formula CxHyOz
requires sufficient oxygen gas and will produce carbon dioxide and water:
CxHyOz + O2 → CO2
+ H2O
The products carbon dioxide and
water will be the same regardless of whether z = 0 or z > 0 or, in other
words, regardless of whether or not the compound burned contains oxygen.
Sample Exercise 10A:
Write a balanced equation for the
combustion of each compound below using the smallest possible whole-number
coefficients:
(a) hexane
(b) propanol
Solution:
(a) 2 C6H14 +
19 O2 → 12 CO2
+ 14 H2O
(b) 2 C3H7OH
+ 9 O2 → 6 CO2
+ 8 H2O
Section 10-4: Reactions Involving Metals or Metal
Compounds
Neutral metals or compounds
containing metal ions are reactants in the following reactions:
(a) metal + nonmetal → ionic compound (also known as ionic salt)
For example, lithium metal reacts
with nitrogen gas to produce lithium nitride according to the following
balanced equation:
(lithium nitride = combine 3 Li+ and N3-
= Li3N)
6 Li + N2
→ 2 Li3N
(b) metal + water → hydrogen gas + metal cation
+ hydroxide ion
For example, sodium metal reacts
with water according to the following balanced equation:
(be sure that the metal cation has
the correct charge)
2 Na + 2 H2O
→ H2
+ 2 Na+ + 2 OH-
(note that the coefficient 2 in front of the Na+ is added to ensure that the total positive charge equals the total negative charge on the right side of the equation)
(c) metal oxide + water → metal cation + hydroxide
ion
For example, potassium oxide reacts
with water as follows:
(potassium oxide = combine 2 K+ and O2-
= K2O)
K2O
+ H2O → 2 K+
+ 2 OH-
(d) metal carbonate → metal oxide + carbon dioxide
For example, magnesium carbonate
decomposes when heated to produce magnesium oxide and carbon dioxide according
to the following balanced equation:
(magnesium carbonate = combine Mg2+ and CO32-
= MgCO3,
magnesium oxide = combine Mg2+ and O2- = MgO)
MgCO3
→ MgO + CO2
Sample Exercise 10B:
Write a balanced equation for each
reaction described below using the smallest possible whole-number coefficients:
(a) aluminum
metal reacts with solid iodine
(b) calcium
metal reacts with water
(c) strontium
oxide reacts with water
(d) sodium
carbonate decomposes upon heating
Solution:
(a) (product
is aluminum iodide = combine Al3+ and 3 I- = AlI3)
2 Al + 3 I2
→ 2 AlI3
(b) (metal
cation produced is Ca2+)
Ca + 2 H2O
→ H2
+ Ca2+ + 2 OH-
(c) (strontium
oxide = combine Sr2+ and O2- = SrO)
SrO + H2O → Sr2+
+ 2 OH-
(d) (sodium
carbonate = combine 2 Na+ and CO32- = Na2CO3,
product is sodium
oxide = combine 2 Na+ and O2- = Na2O)
Na2CO3
→ Na2O
+ CO2
Section 10-5: Aqueous Ionic Compounds and Molarity of Ions
When a solid ionic compound is
dissolved in water, the ions separate from each other and become surrounded by
water molecules in the aqueous solution.
For example, KI separates in water into one K+ cation and one I- anion per formula unit, while
(NH4)2SO4 separates
in water into two NH4+ cations
and one SO42- anion per formula unit. A molarity
written on the bottle of an aqueous ionic compound will be accompanied by the
formula of the neutral ionic compound but will not specify the molarity of individual ions in the solution. As such, if we wish to know the molarity of each type of ion in the solution, we must
translate the information on the bottle using the number of cations
and anions per formula unit of the ionic compound. For example, in a bottle labeled 0.026 M Na3PO4,
the molarity of Na+ will be 3 x 0.026 M =
0.078 M Na+ because there are 3 Na+ cations
per formula unit. The molarity of PO43- will be 1 x 0.026 M
= 0.026 M PO43- since there is one PO43-
anion per formula unit.
Sample Exercise 10C:
What is the molarity
of each ion in the following solutions?
(a) 1.2 M CaCl2
(b) 0.31 M Al(NO3)3
Solution:
(a) 1 x 1.2 M = 1.2 M Ca2+
and 2 x 1.2 M = 2.4 M Cl-
(b) 1 x 0.31 M = 0.31 M Al3+
and 3 x 0.31 M = 0.93 M NO3-
Section 10-6: Solubility Rules, Precipitation
Reactions, and Net Ionic Equations
A solid ionic compound that
dissolves significantly in water is said to be soluble. A solid ionic compound that does not
dissolve to a significant extent in water is said to be insoluble. The following general solubility rule
indicates several groups of ionic compounds that will be soluble:
Ionic compounds containing a Group 1 alkali metal cation,
the ammonium cation (NH4+), or the
nitrate anion (NO3-) are soluble and, therefore, will
exist as separate ions and not as a solid in the presence of water.
Note that there are other ionic compounds which do not include any of the ions in the
solubility rule above but will also still be soluble in some cases. For example, ionic compounds containing
the anions Cl-, Br-, I-,
or SO42- combined with cations
other than NH4+ or Group 1 cations
may be soluble in some cases and insoluble in others.
When two different ionic compound
solutions are mixed, a reaction will occur if cations
from one solution can combine with anions from the other solution to form a
solid precipitate that is
insoluble. Since a solid
precipitate is observed when the solutions Mg(NO3)2
(aq) and K3PO4 (aq) are mixed, we can deduce the formula of the precipitate
by eliminating the combination of cation + anion that
cannot possibly form an insoluble solid precipitate in water according to the
solubility rule in bold print above:
K+
(aq) (Group 1 alkali metal cation) + NO3-
(aq) → no reaction
Mg2+
(aq) + PO43- (aq) → Mg3(PO4)2 (s)
The balanced equation for the
reaction of magnesium cations and phosphate anions is
known as a net ionic equation because
it only includes the ions that are truly involved in the precipitation
reaction:
net ionic equation:
3 Mg2+ (aq) + 2 PO43-
(aq) → Mg3(PO4)2
(s)
The K+ cations and NO3- anions are known as spectator ions because, although they
are present in the solution, they do not actually take part in the reaction
and, thus, are omitted from the net ionic equation.
If neither combination of cation + anion can form an insoluble solid precipitate, all
ions will continue to exist separately in the mixture of solutions and, thus,
no overall reaction occurs. For
example, when a solution of lithium sulfate is mixed with a solution of
ammonium nitrate, neither combination of cation from
one solution + anion from the second solution can form an insoluble solid
precipitate, so no overall reaction occurs and no solid will be observed:
Li+
(aq) + NO3-
(aq) → no
reaction
NH4+
(aq) + SO42- (aq) → no
reaction
Note that it is possible for both
combinations of cation + anion to yield a
precipitate, in which case a mixture of two different solid precipitates will
be produced in the same reaction.
Sample Exercise 10D:
Only two of the following four
solution mixtures will react to form a precipitate. Indicate which two combinations yield no reaction and also
write a balanced net ionic equation, including states of matter, for the two
combinations that do yield a precipitate:
(i) AgNO3
(aq) + (NH4)2CO3
(aq)
(ii) NaNO3 (aq) + CuSO4 (aq)
(iii) aqueous
ammonium chloride + aqueous lithium nitrate
(iv) aqueous
potassium hydroxide + aqueous nickel(II) nitrate
Solution:
No
reaction will occur for (ii) and (iii) as all combinations of cation + anion
will be soluble. One precipitate
will form in each of (i) and (iv):
(i)
NH4+ (aq) + NO3- (aq) → no reaction
net ionic equation:
2 Ag+ (aq) + CO32-
(aq) → Ag2CO3 (s)
(ii) Na+ (aq) + SO42- (aq)
→ no
reaction
Cu2+ (aq)
+ NO3- (aq) → no reaction
(iii) NH4+ (aq) + NO3- (aq) → no reaction
Li+ (aq) + Cl- (aq) → no reaction
(iv) K+ (aq) + NO3- (aq)
→ no
reaction
net ionic equation:
Ni2+ (aq) + 2 OH- (aq) → Ni(OH)2 (s)
Section
10-7: Acid + Base Neutralization
Reactions and Molecular Equations
The
reaction of an aqueous acid with an aqueous metal hydroxide can be considered a
neutralization reaction because the acidic H+ ions from the acid
solution and the basic OH- ions from the metal hydroxide solution
combine to form neutral water.
Although these reactions can be written as net ionic equations, we can
also write a molecular equation with
spectator ions included as follows:
acid (aq) +
metal hydroxide (aq) → water (l) + ionic compound (or ionic salt) (aq)
The
correct formula of the ionic compound (or ionic salt) produced is a combination
of the metal cation from the metal hydroxide and the anion from the acid. For example, in the reaction of
sulfuric acid with sodium hydroxide, the Na+ cations from the NaOH
combine with the SO42- anions from the H2SO4
to yield the formula Na2SO4 in the balanced molecular
equation:
H2SO4 (aq) + 2 NaOH (aq) → 2 H2O (l) + Na2SO4
(aq)
Note
that it is possible in some cases for the ionic compound produced to be an
insoluble solid rather than aqueous.
Sample
Exercise 10E:
Write
a balanced molecular equation, including states of matter, for the
neutralization reaction between solutions of acetic acid and calcium hydroxide.
Solution:
For
neutralization reactions, we will write acetic acid as HCH3COO:
2 HCH3COO (aq) + Ca(OH)2 (aq) → 2 H2O (l) + Ca(CH3COO)2
(aq)
Section
10-8: Experiment – Acid +
Base Titration
In a
titration experiment, a precisely
measured volume of one reactant solution is added dropwise from a buret (or
burette) to a second reactant solution until the stoichiometric equivalence
point of the reaction has been reached.
When the stoichiometric equivalence point has been reached, neither
reactant is in excess and, therefore, we can use the mole ratio from the
balanced chemical reaction in stoichiometry calculations to determine the
unknown molarity of one of the reactants.
One
common titration experiment utilizes an aqueous acid + aqueous metal hydroxide
neutralization reaction. An acid
of known molarity and volume is placed in an Erlenmeyer flask along with a few
drops of phenophthalein indicator, which will be colorless in the acid
solution. A metal hydroxide
solution with unknown molarity is then added dropwise from a buret until the
phenolphthalein indicator in the mixture obtains a faint pink color signifying
that the endpoint has been reached.
Although this pink endpoint is caused by a very slight excess of basic
hydroxide ions from the final drop of metal hydroxide solution that was added,
we can assume that the exact stoichiometric equivalence point has been reached
and, therefore, that neither the acid nor the metal hydroxide is in excess at
that point.
After
calculating the known moles of the acid, we can use the mole ratio from the
balanced molecular equation to calculate the unknown moles of the metal
hydroxide added from the buret. We
then divide by the volume in liters of the metal hydroxide solution added from
the buret to obtain the unknown molarity of the metal hydroxide solution. For example, if we start with 16.7 mL
of 0.116 M hydrochloric acid in the Erlenmeyer flask and 14.9 mL of sodium
hydroxide solution is required to titrate the acid (reach the equivalence
point), the unknown molarity of the sodium hydroxide solution can be determined
as follows:

Note
that the calculated molarity of the NaOH solution may differ from the true
value due to experimental error.
For example, if extra drops of NaOH solution are inadvertantly added
from the buret, the volume recorded for NaOH solution added will be too high. Since we would divide by too large a
volume of NaOH solution in the final step, the calculated molarity of NaOH
solution would be erroneously low.
If
we know the volume and molarity of the acid in the Erlenmeyer flask as well as
the molarity of the metal hydroxide solution being added from the buret, we can
calculate the volume of metal hydroxide solution required for the titration, as
demonstrated in the following problem:
Sample
Exercise 10F:
How
many milliliters of 0.019 M strontium hydroxide are required to titrate 24 mL
of 0.026 M nitric acid?
Solution:

Chapter 10 Practice Exercises and Review Quizzes:
10-1) Write
a balanced equation for the combustion of each compound below using the
smallest possible whole-number coefficients:
(a) heptanol
(b) ethane
Click for Solution
10-1) (a)
2 C7H15OH + 21 O2 → 14 CO2 + 16 H2O
(b) 2
C2H6 + 7 O2 → 4 CO2 + 6 H2O
10-2) Write a balanced equation for each reaction described below using the smallest possible whole-number coefficients:
(a)
barium metal reacts with oxygen gas
(b) rubidium metal reacts with water
(c) cesium oxide reacts with water
(d) calcium carbonate decomposes upon heating
Click for Solution
10-2)
(a) (product
is barium oxide = combine Ba2+ and O2- = BaO)
2 Ba + O2 → 2 BaO
(b)
2 Rb + 2 H2O → H2
+ 2 Rb+ + 2 OH-
(c) (cesium
oxide = combine Cs+ and O2- = Cs2O)
Cs2O
+ H2O → 2 Cs+
+ 2 OH-
(d) (calcium
carbonate = combine Ca2+ and CO32- = CaCO3,
CaCO3
→ CaO + CO2
10-3) What
is the molarity of each ion in the following
solutions?
(a) 0.18 M (NH4)2CO3
(b) 0.014 M FeBr3
Click for Solution
10-3) (a)
2 x 0.18 M = 0.36 M NH4+ and 1 x 0.18 M = 0.18 M CO32-
(b) 1
x 0.014 M = 0.014 M Fe3+ and 3 x 0.014 M = 0.042 M Br-
10-4) Only
two of the following four solution mixtures will react to form a
precipitate. Indicate which two
combinations yield no reaction and also write a balanced net ionic equation,
including states of matter, for the two combinations that do yield a
precipitate:
(i) aqueous lithium nitrate + aqueous ammonium carbonate
(ii) aqueous
sodium phosphate + aqueous calcium nitrate
(iii) Pb(NO3)2
(aq) + K2SO4 (aq)
(iv) NaI
(aq) + NH4Br (aq)
Click for Solution
10-4) No reaction will occur for (i) and (iv) as all
combinations of cation + anion will be soluble. One precipitate will form in each of (ii) and (iii):
(i)
NH4+ (aq) + NO3- (aq) → no reaction
Li+ (aq) +
CO32- (aq) → no
reaction
(ii) Na+ (aq) + NO3- (aq)
→ no
reaction
net ionic equation:
3 Ca2+ (aq) + 2 PO43-
(aq) → Ca3(PO4)2 (s)
(iii) K+ (aq) + NO3- (aq) → no reaction
net ionic
equation: Pb2+ (aq) + SO42- (aq)
→ PbSO4 (s)
(iv) NH4+ (aq) + I- (aq) → no reaction
Na+ (aq) + Br- (aq) → no reaction
10-5) Write a
balanced molecular equation, including states of matter, for the neutralization
reaction between solutions of sulfuric acid and lithium hydroxide.
Click for Solution
10-5) H2SO4 (aq) + 2 LiOH (aq) → 2 H2O (l) + Li2SO4
(aq)
10-6)
If 13 mL of 0.24 M barium hydroxide solution is required to titrate 28 mL of
acetic acid solution, what is the molarity of the acetic acid solution?
Click for Solution
10-6)
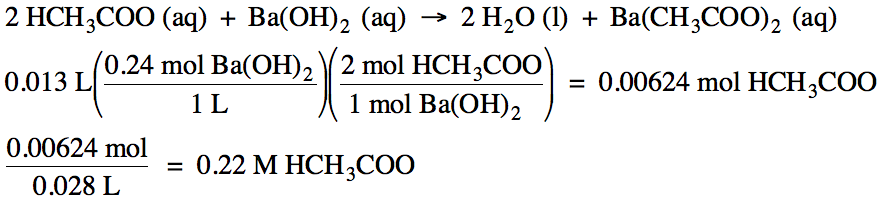
10-7)
How many milliliters of 0.0164 M hydrochloric acid can be titrated with 12.5 mL
of 0.0214 M calcium hydroxide?
Click for Solution
10-7)