Chapter 11: Molecular Geometry and Polarity of
Molecules
Section 11-2: Polarity of Molecules
Chapter 11 Practice Exercises and Review Quizzes
Section 11-1: Molecular Geometry: Using VSEPR Theory to Determine
Three-Dimensional Shapes and Bond Angles
Diatomic molecules and ions are
linear, regardless of the number of covalent bonds between the atoms. For molecules or ions with three or
more bonded atoms, the Lewis structure indicates which specific atoms are bonded
together and the number of bonds between the atoms. However, the Lewis structure does not necessarily provide an
accurate depiction of the three-dimensional shape and bond angles in the
molecule or ion.
Valence-Shell Electron-Pair
Repulsion (VSEPR) Theory suggests
that the valence electron domains comprised of covalent bonds and lone pairs adjacent to the central atom in a
molecule or ion will be arranged in a three-dimensional shape that minimizes
electron repulsion between the bonds and lone pairs. We will use the VSEPR notation ABxEy to categorize molecules and
ions, where A represents the center atom, x represents the number of outer
atoms (B) bonded to the center atom A, and y represents the number of lone
pairs (E) on the center atom A.
Once we know the VSEPR notation for an atom or ion, we can determine the
three-dimensional shape and bond angles.
When we draw three-dimensional sketches of molecules or ions on paper,
we will use solid straight lines to represent bonds oriented in the plane of
the paper, dashed wedges to represent bonds oriented back behind the plane of
the paper, and solid wedges to represent bonds oriented forward out of the
paper. We will also only include
lone pairs on the center atom in sketches, but not lone pairs on the outer atoms as these generally do not affect the shape according
to VSEPR Theory:
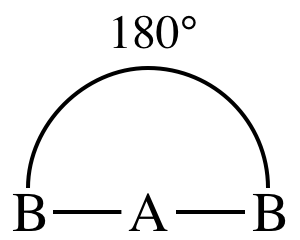
I. AB2E0 = AB2 = two
outer atoms bonded to center atom + no lone pairs on center atom = molecule is linear:
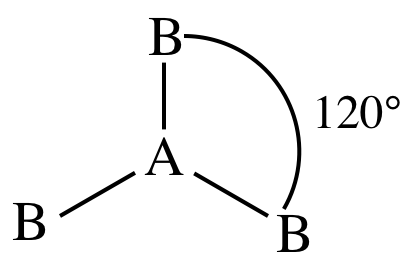
II. AB3E0 = AB3 = three
outer atoms bonded to center atom + no lone pairs on center atom = molecule is trigonal planar:
III. AB2E1 = two outer atoms bonded to center
atom + one lone pair on center atom = molecule is bent:
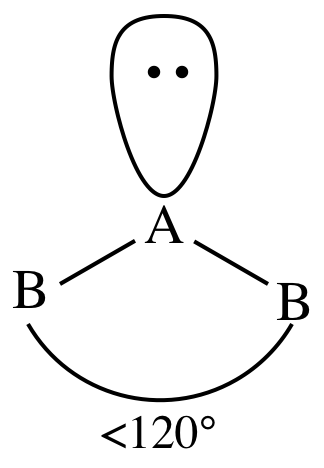
The bond angle is decreased below
120° because the lone pair has a slightly greater repulsive effect than the
electrons in the covalent bonds between A and B.
IV. AB4E0 = AB4 = four
outer atoms bonded to center atom + no lone pairs on center atom = tetrahedral:
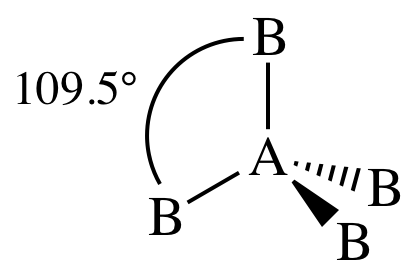
V. AB3E1 = three outer atoms bonded to
center atom + one lone pair on center atom = trigonal pyramidal:
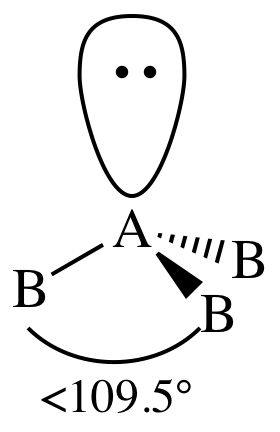
The bond angles are decreased below
109.5° because the lone pair has a slightly greater repulsive effect than the
electrons in the covalent bonds between A and B.
VI. AB2E2 = two outer atoms bonded to center
atom + two lone pairs on center atom = bent:
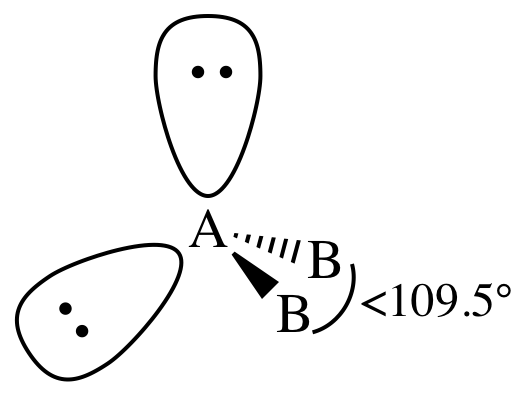
In the AB2E2
= bent case, the extra electron repulsion caused by the second lone pair on the
center atom may cause the B-A-B bond angle to decrease even further below
109.5° than the decrease expected in the AB3E1 = trigonal pyramidal case with only one lone pair on the
center atom.
In contrast to the Lewis
structures, our three-dimensional sketches will neglect the differences between
and, thus, not distinguish among single, double, and triple bonds, as
demonstrated in the following problem:
Sample Exercise 11A:
Draw the Lewis structure, name the
molecular geometry (shape), draw a three-dimensional sketch, and indicate the
bond angle for each of the following molecules and ions:
(a) CO2
(b) NH3
(c) CO32-
(d) CH4
(e) NO2-
(f) H2O
Solution:
(a)
Lewis structure:
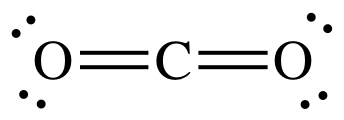
AB2
= linear
3-D sketch:
(no need
to distinguish between single v. double v. triple bonds in sketch)
(b)
Lewis structure:
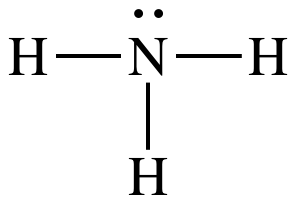
AB3E1
= trigonal pyramidal
3-D sketch:
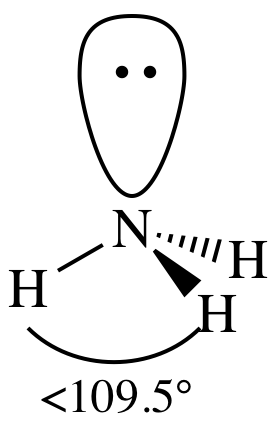
(c)
Lewis structure:
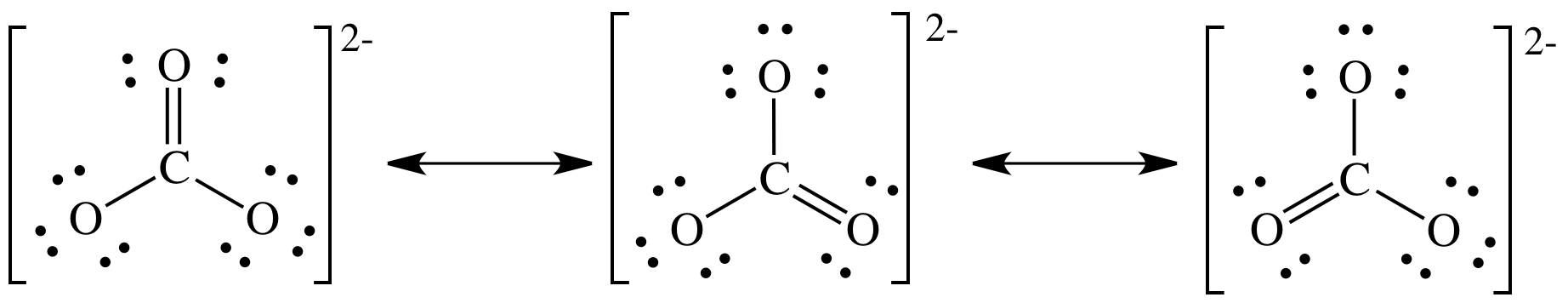
Note that we do not actually need
to draw all the resonance structures to determine the shape as we can see from
any one of the three resonance structures that the carbonate ion = AB3
= trigonal planar.
3-D sketch:
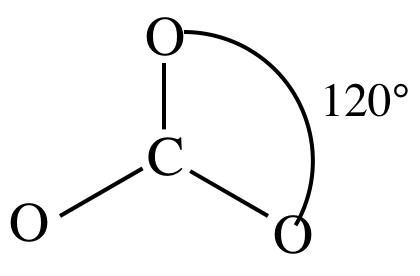
(d)
Lewis structure:
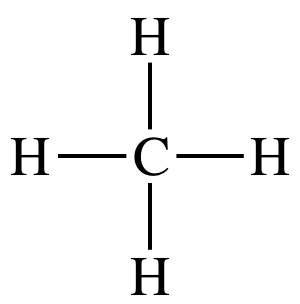
AB4
= tetrahedral
3-D sketch:
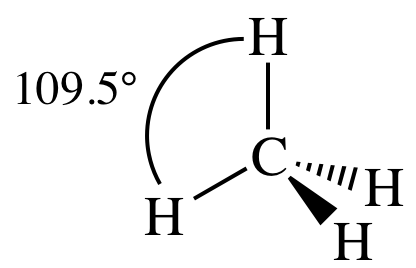
(e)
Lewis structure:
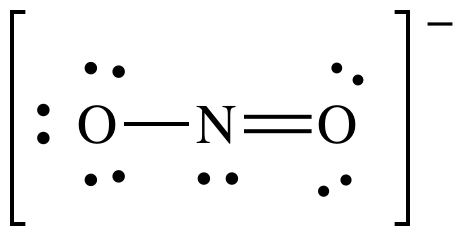
AB2E1
= bent (no need to show all resonance structures to determine shape)
3-D sketch:
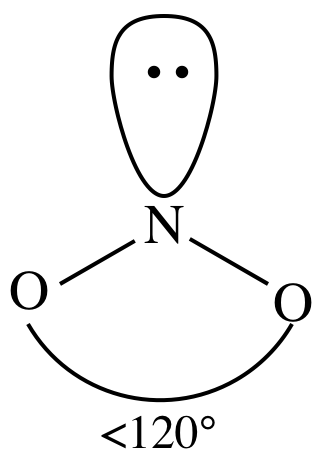
(f)
Lewis structure:
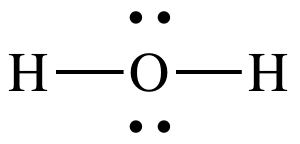
AB2E2
= bent
3-D sketch:
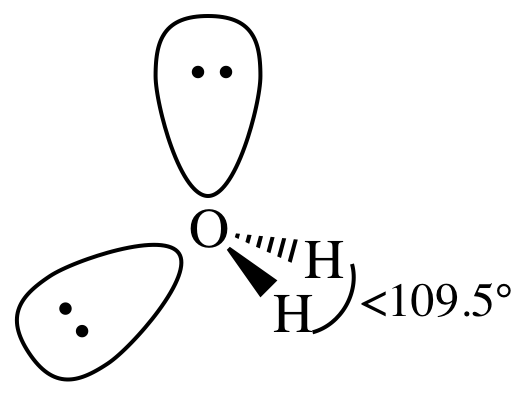
For molecules or ions with more
than one center atom, we can describe the shape in the region of each center
atom as demonstrated in the following problem:
Sample Exercise 11B:
Draw the Lewis structure for acetic
acid. Name the molecular geometry
and indicate the bond angles in the region of each center atom.
Solution:
Acetic acid = CH3COOH. All organic acids with the ending COOH
have a group of atoms (in this case CH3) single-bonded to the carbon
in the COOH. The carbon in the
COOH is double-bonded to one oxygen and single-bonded
to the second oxygen, with the hydrogen in the COOH single-bonded to the second
oxygen. Therefore, the Lewis
structure is:
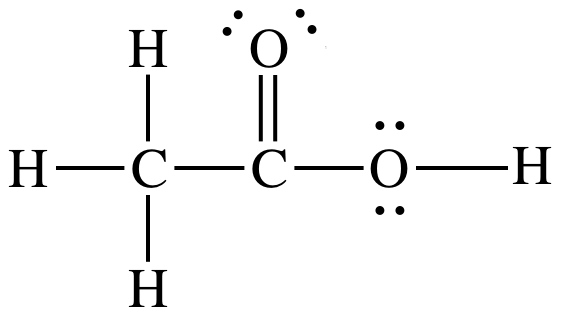
carbon on the left = AB4 = tetrahedral, bond
angles = 109.5°
carbon in center = AB3 = trigonal
planar, bond angles = 120°
oxygen on right = AB2E2 = bent, bond
angle = <109.5°
Section 11-2: Polarity of Molecules
In a polar covalent bond, the
electrons will be more attracted toward the more electronegative atom. We can indicate the direction in which
the electrons are shifted in a polar covalent bond by placing a bond dipole
arrow parallel to the bond in a sketch of the molecule, with the head of the
arrow closer to the more electronegative atom and the + end of the arrow closer
to the less electronegative atom.
For the molecule BrF, the head of the bond
dipole arrow will be closer to the more electronegative F atom and the + end of
the bond dipole arrow will be closer to the less electronegative Br atom:
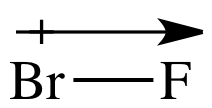
A dipole moment (μ) is
essentially a measurement of the overall net shift of electrons toward a
particular direction in a neutral molecule. A molecule with no identifiable direction toward which the
electrons are shifted is said to be a nonpolar
molecule with zero dipole moment (μ = 0). A molecule with an identifiable
direction toward which the electrons are shifted is said to be a polar molecule
with a dipole moment greater than zero (μ >
0).
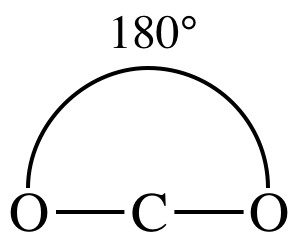
In a sketch of the linear carbon
dioxide molecule, the heads of the two bond dipole arrows will be closer to the
more electronegative outer oxygen atoms:
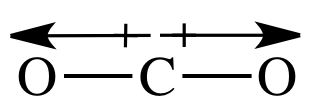
However, since the two bond dipole
arrows essentially cancel each other out because they are equal in magnitude
but are oriented in opposite directions, carbon dioxide is a nonpolar molecule (μ = 0).
In a sketch of the bent water
molecule, the head of the two bond dipole arrows will be closer to the more
electronegative center oxygen atom:
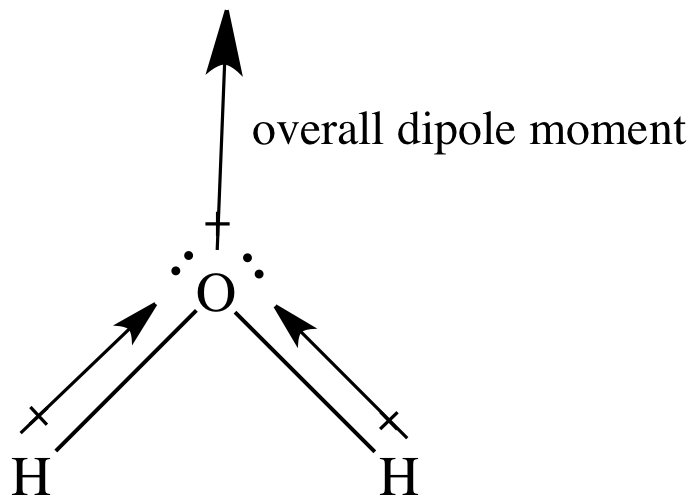
In the case of water, the two bond
dipoles arrows are equal in magnitude but do not cancel each other out. We see that there is an overall net
shift of electrons and, therefore, a dipole moment oriented toward the oxygen
end of the molecule. Thus, water
is a polar molecule (μ >
0).
The following general guideline
indicates which categories of molecules will be nonpolar
and which categories of molecules will be polar:
Molecules with no lone pairs on the center atom will generally be nonpolar if all the outer atoms are the same element. Molecules with one or more lone pairs
on the center atom and molecules with outer atoms that are different elements
will generally be polar.
For each different category, the
table below summarizes the molecular geometry (shape), bond angle, and whether
the molecule is polar or nonpolar:
|
VSEPR Notation |
Name of Molecular Geometry (Shape) |
Bond Angle |
Polar or Nonpolar
Molecule? |
|
AB2 |
linear |
180° |
nonpolar* |
|
AB3 |
trigonal planar |
120° |
nonpolar* |
|
AB2E1 |
bent |
<120° |
polar |
|
AB4 |
tetrahedral |
109.5° |
nonpolar* |
|
AB3E1 |
trigonal pyramidal |
<109.5° |
polar |
|
AB2E2 |
bent |
<109.5° |
polar |
*unless outer atoms are
different elements
Sample Exercise 11C:
Draw the Lewis structure, name the
molecular geometry (shape), draw a three-dimensional sketch, indicate the bond
angle, and state whether each of the following molecules is polar or nonpolar:
(a) BCl3
(b) CH2F2
(c) HCN
(d) SCl2
(e) PF3
(f) HNO
Solution:
(a)
Lewis structure:
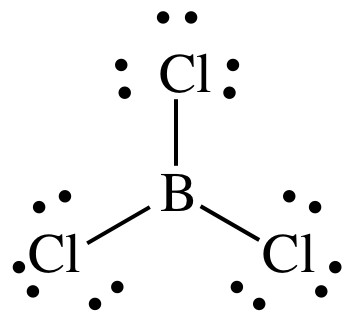
AB3
= trigonal planar
3-D sketch:
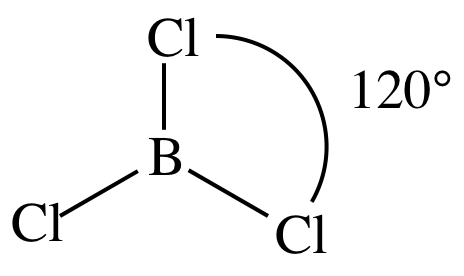
nonpolar molecule
(b)
Lewis structure:
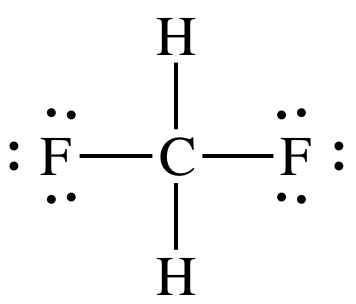
AB4
= tetrahedral
3-D sketch:
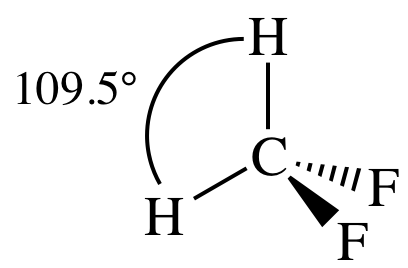
polar molecule (different outer elements)
(c)
Lewis structure:
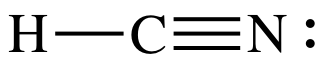
AB2 = linear
3-D sketch:
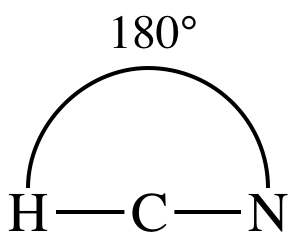
polar molecule (different outer elements)
(d)
Lewis structure:
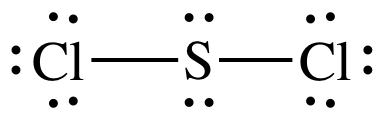
AB2E2
= bent
3-D sketch:
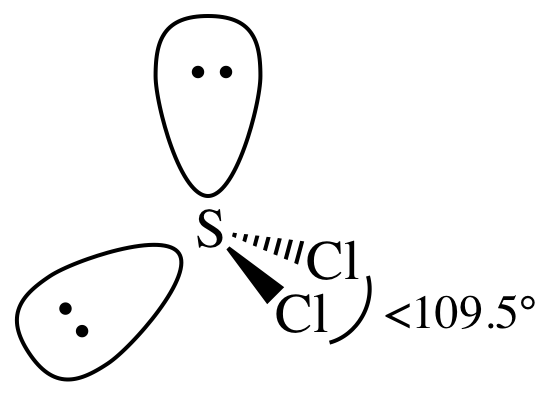
polar molecule
(e)
Lewis structure:
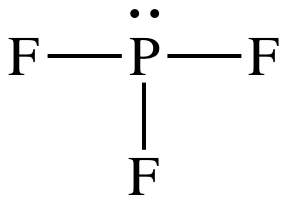
AB3E1
= trigonal pyramidal
3-D sketch:
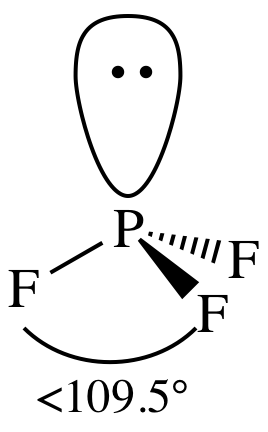
polar molecule
(f)
Lewis structure:
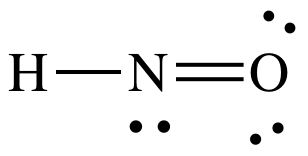
AB2E1
= bent
3-D sketch:
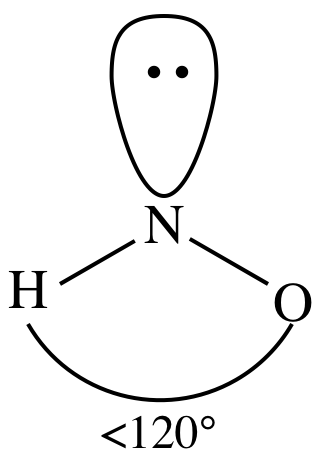
polar molecule
Chapter 11 Practice Exercises and Review Quizzes:
11-1) Draw the Lewis structure,
name the molecular geometry (shape), draw a three-dimensional sketch, and
indicate the bond angle for each of the following molecules and ions:
(a) PH3
(b) NH2-
(c) NO3-
(d) O3
(e) NH4+
(f) NO2+
Click for Solution
11-1)
(a)
Lewis structure:
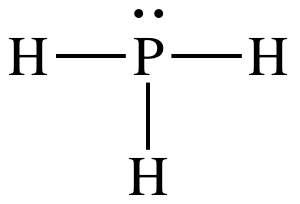
AB3E1
= trigonal pyramidal
3-D sketch:
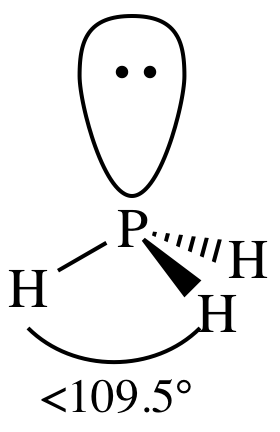
(b)
Lewis structure:
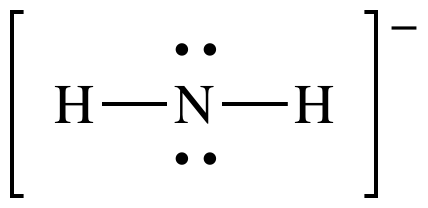
AB2E2
= bent
3-D sketch:
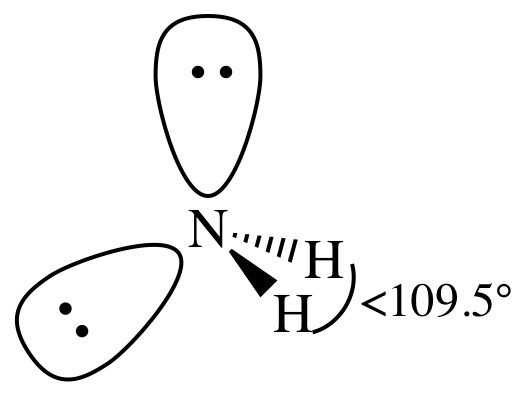
(c)
Lewis structure:
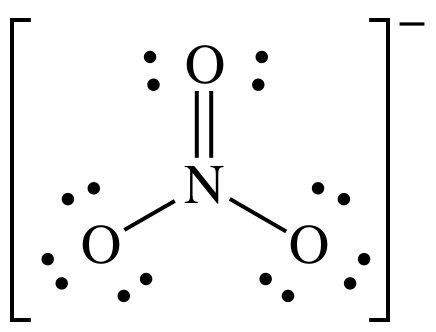
AB3
= trigonal planar
3-D sketch:
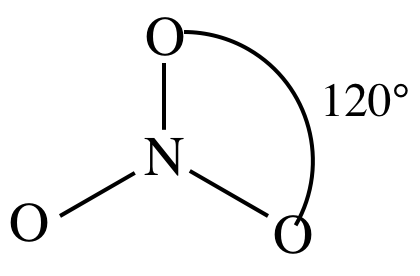
(d)
Lewis structure:
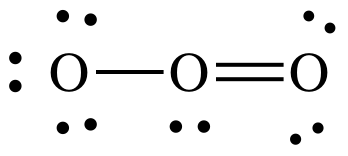
AB2E1
= bent
3-D sketch:
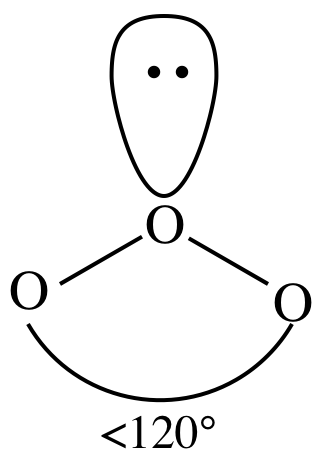
(e)
Lewis structure:
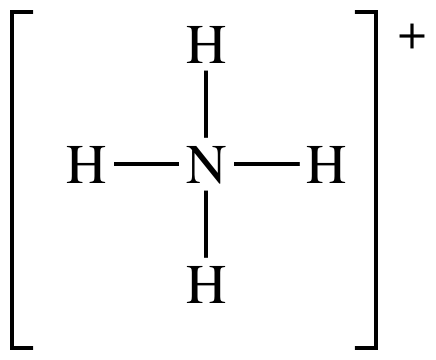
AB4
= tetrahedral
3-D sketch:
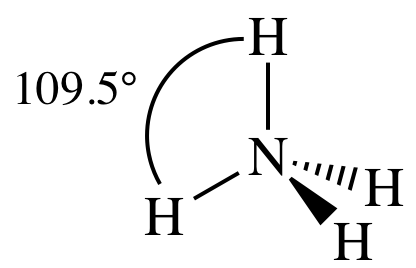
(f)
Lewis structure:
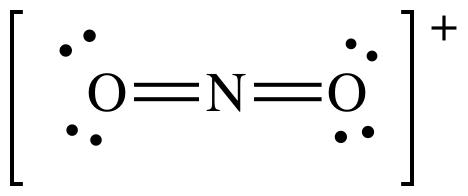
AB2
= linear
3-D sketch:
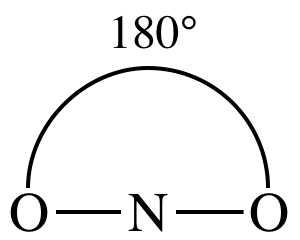
11-2) Draw the Lewis structure,
name the molecular geometry (shape), draw a three-dimensional sketch, indicate
the bond angle, and state whether each of the following molecules is polar or nonpolar:
(a) SiH4
(b) HOBr
(c) CS2
(d) NI3
(e) ClNO
(f) BF3
Click for Solution
11-2)
(a)
Lewis structure:
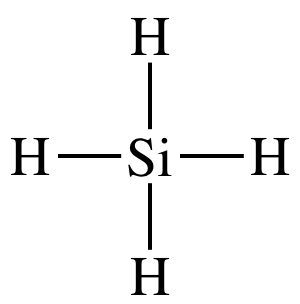
AB4
= tetrahedral
3-D sketch:
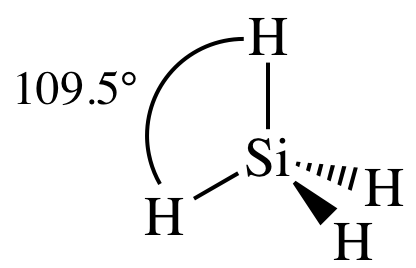
nonpolar molecule
(b)
Lewis structure:
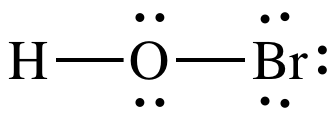
AB2E2
= bent
3-D sketch:
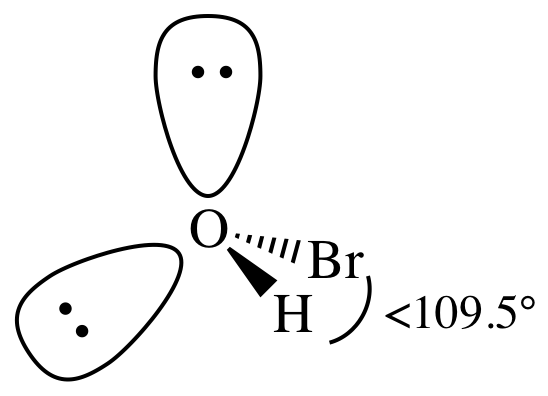
(c)
Lewis structure:
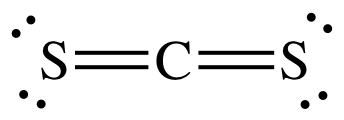
AB2
= linear
3-D
sketch:
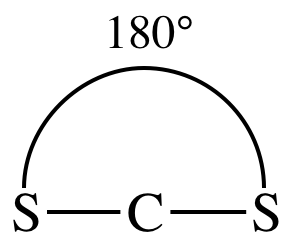
nonpolar molecule
(d)
Lewis structure:
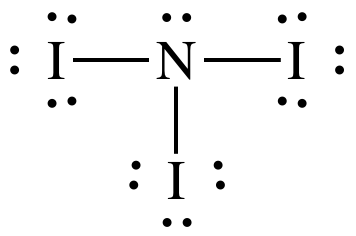
AB3E1
= trigonal pyramidal
3-D sketch:
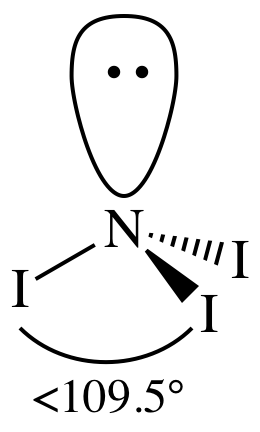
polar molecule
(e)
Lewis structure:
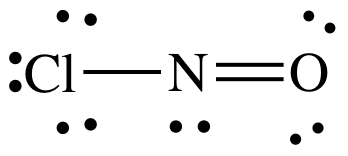
AB2E1
= bent
3-D sketch:
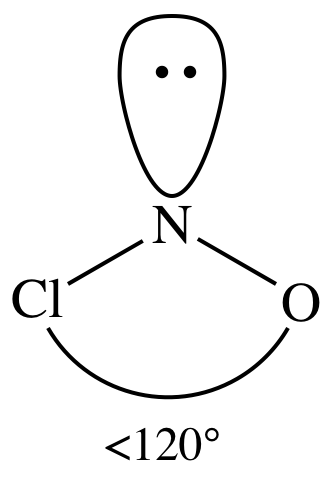
polar molecule
(f)
Lewis structure:
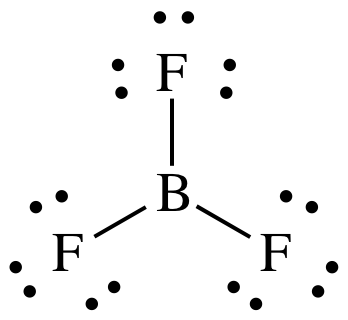
AB3
= trigonal planar
3-D sketch:
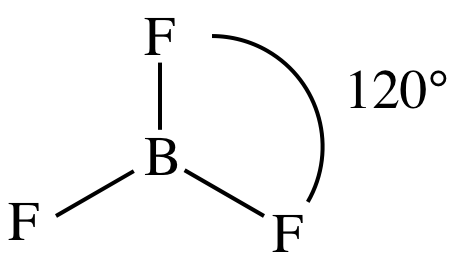
nonpolar molecule
