Chapter 12: Crystal Types and Intermolecular Forces
Section 12-2: Intermolecular Forces
Section 12-3: "Like Dissolves Like" and Solubility
Chapter 12 Practice Exercises and Review Quizzes
Section 12-1: Crystal Types
Many different substances in the
solid state can be categorized into one of the following crystal types:
I. Network Covalent
In a network covalent crystal, the
atoms are held together in a continuous three-dimensional array of covalent
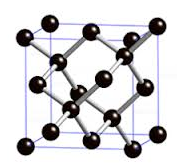
bonds. For example, the carbon
atoms in the network covalent C(diamond) are each
bonded tetrahedrally to four neighboring carbon atoms
as follows:
Other network covalent substances
include SiC and SiO2. Network covalent crystals generally
have the following properties:
1. High melting point.
2. Physically hard.
3. Poor conductor of electricity. For a substance to conduct electricity, charged particles
must have the ability to move freely throughout the sample. In the case of C(diamond),
SiC, and SiO2, the negatively-charge
electrons in the covalent bonds are localized between two particular atoms and,
therefore, do not have the ability to move freely throughout the crystal. As such, C(diamond),
SiC, and SiO2 are poor conductors of
electricity.
C(graphite)
is also considered network covalent because the carbon atoms are covalently
bonded in a continuous trigonal planar pattern to
form layers:
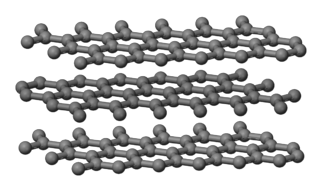
In contrast to the physically hard C(diamond), SiC, and SiO2,
layers in a sample of C(graphite) will more readily flake off. Each carbon atom in C(graphite)
will use 3 of its 4 available valence electrons to form covalent bonds with 3
neighboring carbon atoms. However,
all the extra valence electrons will be delocalized between the layers of
carbon and, thus, able to move freely throughout the crystal. As such, C(graphite)
will be a good conductor of electricity.
II. Ionic
Ionic crystals contain a continuous
three-dimensional array of positively-charged metal cations or polyatomic cations
such as ammonium (NH4+) and negatively-charged nonmetal
anions or polyatomic anions. Solid
ionic crystals generally have the following properties:
1. High melting point.
2. Physically brittle.
3. Poor conductor of electricity
because the charged cations and anions are held
firmly in place and not able to move freely throughout the crystal (cations and anions are immobile).
Despite still being in close
contact, the charged cations and anions in a liquid
(molten) ionic compound are able to move freely throughout the sample (cations and anions are mobile). Therefore, a liquid ionic compound is a
good conductor of electricity. In
an aqueous ionic compound, the individual cations and
anions are surrounded by water molecules and completely separated from each
other. Because these charged ions
are able to move freely throughout the solution, an aqueous ionic compound is
also a good conductor of electricity.
One aspect of Coulomb's Law
essentially suggests that the attraction between two oppositely-charged
particles will be greater when the magnitudes of the particles' charges are greater. Greater Coulombic attraction between the cations and anions in an ionic solid will result in a
higher melting point. When
comparing the melting points of ionic solids, a compound with a larger sum of one cation's charge magnitude + one anion's charge magnitude
will often have the higher melting point (although ionic radii can also significantly affect melting point), as demonstrated in the following
problem:
Sample Exercise 12A:
Rank the compounds CsBr, MgO, and SrCl2
from lowest to highest melting point and justify your answer.
Solution:
First, we determine the sum of one cation's charge magnitude + one anion's charge magnitude for each
compound:
CsBr = Cs+ and Br-, sum of charge
magnitudes = 1 + 1 = 2
MgO = Mg2+ and O2-, sum of charge
magnitudes = 2 + 2 = 4
SrCl2
= Sr2+ and Cl-, sum of charge
magnitudes = 2 + 1 = 3
CsBr has the lowest sum and, therefore, the lowest melting point. MgO has the highest sum and, therefore, the highest melting point. As such, the ranking from lowest to highest melting point is CsBr < SrCl2 < MgO.
III. Metallic
Metallic crystals are often
described as essentially being a three-dimensional array of metal cations in a sea of delocalized electrons. Metallic crystals generally have the following properties:
1. Broad
range of melting points, but all metals except mercury (Hg) are
solids at room temperature.
2. Physically shiny, malleable, and
ductile.
3. Good conductor of electricity
because some electrons from each atom are delocalized and can move freely
throughout the crystal.
Liquid metals also have delocalized
electrons and, therefore, are good conductors of electricity.
IV. Molecular
Many substances that are not
network covalent, ionic, or metallic form molecular crystals in the solid
state. Molecular crystals
generally have the following properties:
1. Low melting points because the
intermolecular forces between molecules are relatively weak. Generally lower melting points than
network covalent, ionic, or metallic crystals.
2. Physically not hard.
3. Poor conductor of electricity
because electrons within the molecules are localized and are not able to move
freely throughout the crystal.
Molecular substances in the liquid
state are poor conductors of electricity because electrons within the molecules
are localized and are not able to move freely throughout the sample. Molecular substances in the liquid
state also generally have lower boiling points than ionic or metallic
substances.
Sample Exercise 12B:
Categorize each substance below as
being network covalent, ionic, metallic, or molecular:
(a) (NH4)2CO3
(b) Ni
(c) SiC
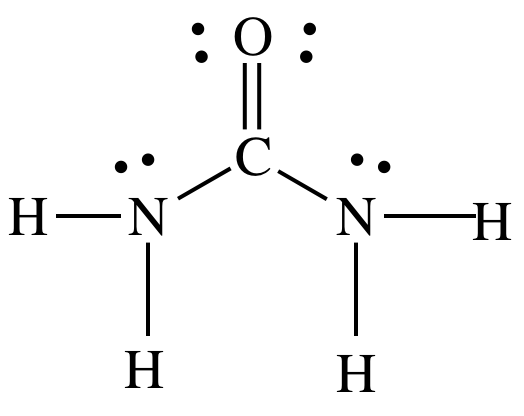
(d) (NH2)2CO
Solution:
(a) NH4+ and
CO32- = ionic
(b) nickel
= metallic
(c) SiC =
network covalent
(d) molecular,
Lewis structure:
Sample Exercise 12C:
Which of the following has the
lowest melting point?
(a) CCl4 (b)
Cu
(c) NaBr
(d) SiO2
Solution:
CCl4 = molecular = low
melting point
Cu = metallic = generally higher
melting point than molecular
NaBr = Na+
and Br- = ionic = high melting point
SiO2 = network covalent
= high melting point
Therefore, (a) CCl4 has
the lowest melting point.
Sample Exercise 12D:
State whether each of the following
is a good or poor conductor of electricity in the solid state:
(a) BaCl2
(b) C(graphite)
(c) Kr
(d) Mn
Solution:
(a) Ba2+ and Cl- = ionic = poor conductor in solid state because cations and anions are immobile (but
good conductor in liquid or aqueous state because cations and anions are mobile)
(b) network covalent but, unlike other network covalent crystals, has delocalized electrons
between the planar layers = good conductor in solid state
(c) nonmetal = molecular = poor conductor in solid state because electrons are localized (and also poor conductor in liquid state because electrons are localized)
(d) manganese = metallic = good conductor in solid state because electrons are delocalized (and also good conductor in liquid state because electrons are delocalized)
Section 12-2: Intermolecular Forces
Molecular substances with stronger intermolecular forces (IMFs) will
have higher boiling points because the molecules will be more strongly held
together. The three different
types of IMFs are described below:
I. Hydrogen Bonding
Hydrogen bonding occurs only between molecules containing hydrogen
atoms that are bonded DIRECTLY
to nitrogen, oxygen, or fluorine atoms.
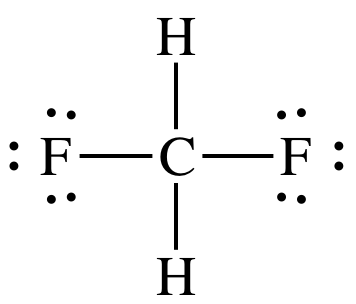
For example, HF is capable of hydrogen bonding, whereas difluoromethane, CH2F2, is not
capable of hydrogen bonding because the H atoms are not bonded directly to the
F atoms, as shown in the following Lewis structure:
Sample Exercise 12E:
Which of the following is not
capable of hydrogen bonding?
(a) C2H5OH
(b) CH3CN
(c) H2O2
(d) N2H4
Solution:
(b) CH3CN (H not bonded
directly to N)
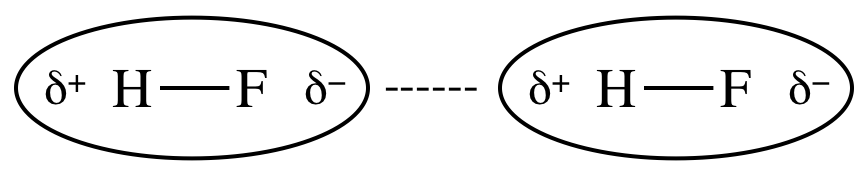
In a sample of HF, the covalent
bond in each molecule is highly polar because of the
large electronegativity difference between H (EN =
2.1) and F (EN = 4.0), leading to a partial negative charge (δ-) on the F
end of the molecule and a partial positive charge (δ+) on the H
end of the molecule. The strong
attraction between the F end of one molecule and the H end of a neighboring
molecule is known as a hydrogen bond and is represented by the a dashed line
below:
Molecular substances that are
capable of hydrogen bonding generally have higher boiling points than molecular
substances that are not capable of hydrogen bonding.
For the majority of substances, the
density of the solid is higher than the density of the liquid because the
molecules in the solid are packed closely together and then move slightly
farther apart in the liquid.
However, the unique combination of small size, bent shape, and ability
to hydrogen bond for H2O molecules allows ice to obtain a crystal
structure wherein the water molecules are separated by a significant distance
by hydrogen bonds, as shown below (red
= oxygen atoms, white = hydrogen atoms):
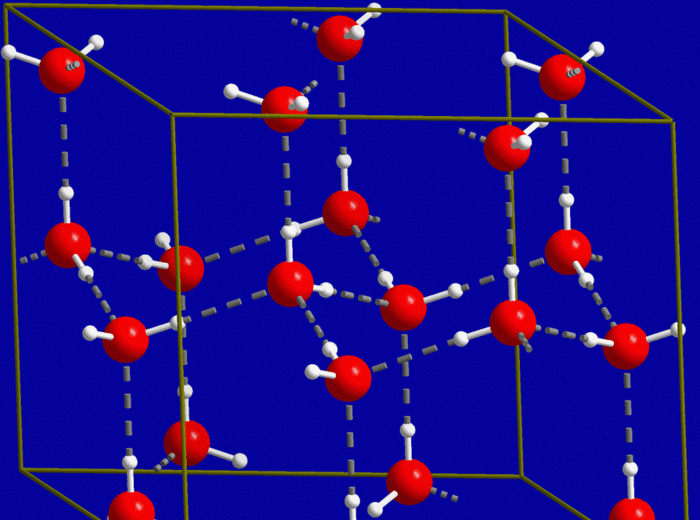
The significant empty space between
molecules in ice results in a lower density for ice than liquid water when the
two are compared at the freezing point of 0°C.
II. London (Dispersion) Forces
Whereas
only a limited number of molecular substances are capable of hydrogen bonding,
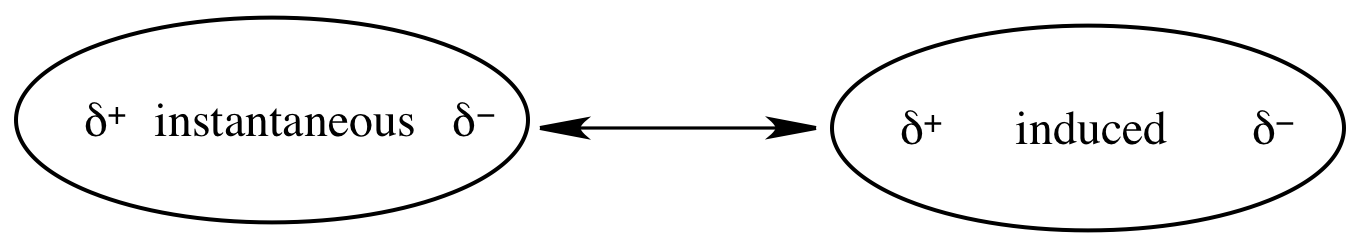
all molecular substances are held together by London (dispersion) forces. When more electrons happen to be on one side of a molecule
than the other at a particular moment in time, an instantaneous dipole is
created with a partial negative charge on the side with more electrons and a
partial positive charge on the side with less electrons. If a second molecule comes in close
proximity to the negative end of the instantaneous dipole, the electron cloud
in the second molecule will be repelled away from the negative end of the
instantaneous dipole. This creates
an induced dipole in the second molecule with a partial positive charge closest
to the first molecule and a partial negative charge furthest from the first
molecule. A London force is the
attraction between the instantaneous dipole and the induced dipole and is
represented by the double-headed arrow below:
Polarizability is
essentially the ease with which the electron cloud can be shifted in a molecule
to create the dipoles necessary for a London force. Molecules with significantly more total
electrons will generally be more polarizable and,
therefore, have stronger London forces.
Molecular substances with stronger London forces will generally have
higher boiling points. Rather than
counting total electrons to compare London forces in different molecular
substances, we can usually obtain the same results by instead comparing molar
masses. Therefore, molecular
substances with significantly larger molar masses will generally have stronger
London forces and higher boiling points.
Sample Exercise 12F:
Explain why Br2 is a
liquid at room temperature, whereas Cl2 is a gas.
Solution:
Both Br2 and Cl2 are molecular substances held together only by London forces. Since Br2 has a
significantly higher total number of electrons (2 x 35 = 70) than Cl2 (2 x 17 = 34), Br2 has London forces strong enough to hold the molecules
together as a liquid, whereas the London forces in Cl2 are not as
strong, so the Cl2 molecules separate into a gas.
III. Dipole-Dipole Forces
Molecular substances containing
polar molecules will have dipole-dipole forces where the partial negative end
of one polar molecule will be attracted to the partial positive end of another
polar molecule. This is similar to
hydrogen bonding, but weaker in strength.
Two molecular substances that are not capable of hydrogen bonding and
that have roughly equal London forces (as suggested by their similar total number of electrons) can have significantly different boiling points if one is polar and,
therefore, is also held together by dipole-dipole forces while the other is nonpolar and, therefore, does not have the extra attraction
due to dipole-dipole forces.
Sample Exercise 12G:
Which will have the higher boiling
point, CO or N2?
Solution:
Both CO and N2 are
molecular substances with the same total number of electrons (CO = 6 + 8 = 14, N2 = 2 x 7 = 14). Therefore, CO and N2 are
expected to have roughly equal London forces. However, CO is polar and,
therefore, has dipole-dipole forces to raise the boiling point above that of
the nonpolar N2, which lacks the extra
attraction of dipole-dipole forces.
Section 12-3: "Like Dissolves Like" and Solubility
A common rule of thumb used to
predict whether or not a solute will dissolve in a solvent is "like dissolves
like":
a. Nonpolar
solutes tend to dissolve in nonpolar solvents. Note that the electronegativity
difference between hydrogen and carbon is so small that we will consider bonds
between hydrogen and carbon to be nonpolar. Therefore, we will consider all
compounds containing only hydrogen and carbon (hydrocarbons) to be nonpolar.
Oils, fats, and gasoline have in common significant hydrocarbon portions
that make them effectively nonpolar. As such, oils, fats, and gasoline will
not dissolve in or mix with water, which is polar, to
any significant extent.
b. Polar solutes tend to dissolve
in polar solvents.
c. Solutes that are capable of
hydrogen bonding tend to dissolve in solvents that are capable of hydrogen
bonding.
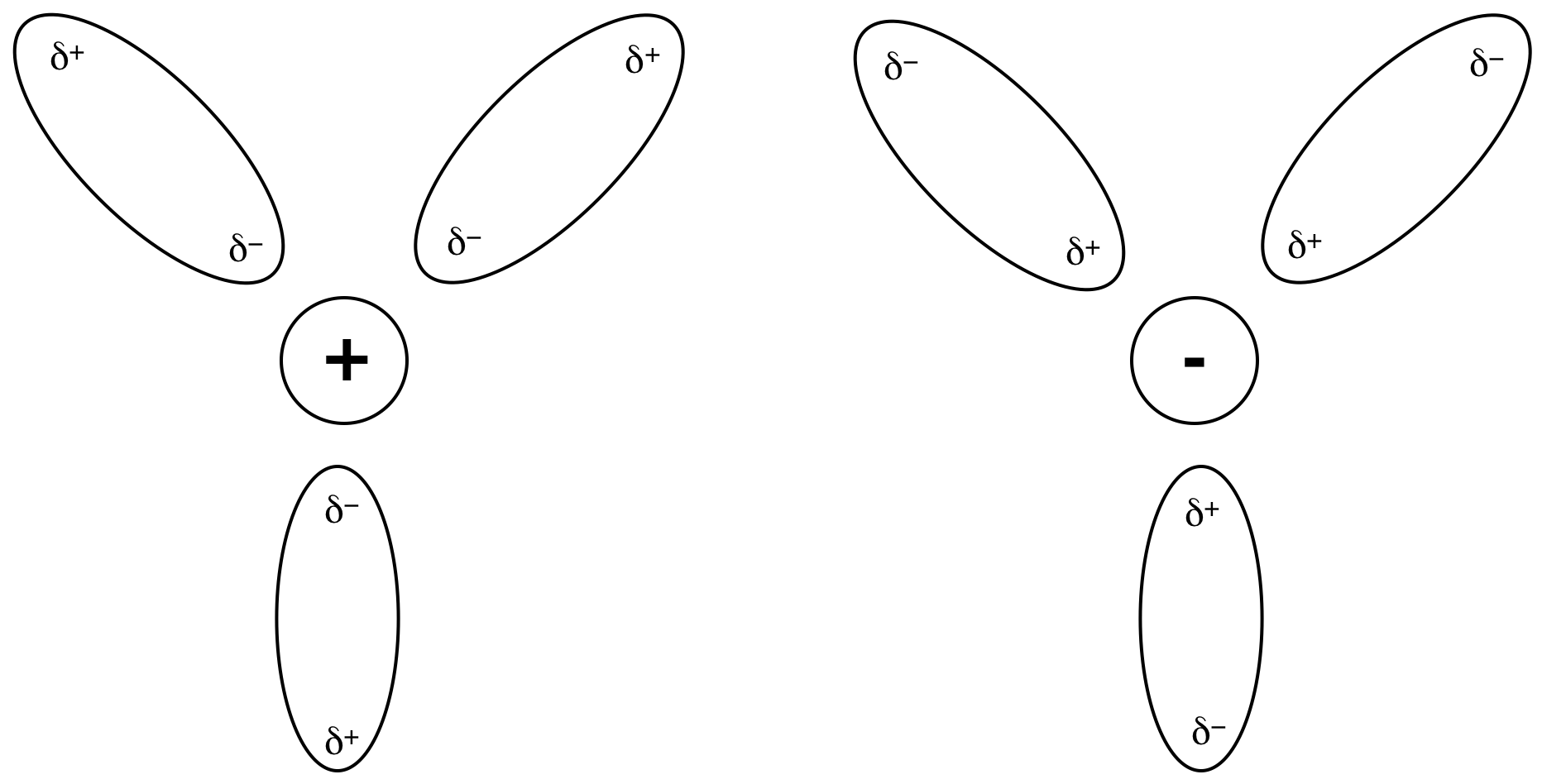
d. Ionic solutes tend to dissolve
in polar solvents. Although
significant energy is required to separate the cations
and anions in the solute during the dissolving process, significant energy is
regained via ion-dipole interactions wherein the partial negative ends of the
polar solvent molecules are attracted to the cations
and the partial positive ends of the polar solvent molecules are attracted to
the anions:
Sample Exercise 12H:
Predict whether each solute below
will dissolve to a greater extent in carbon disulfide or water:
(a) HOCl
(b) I2
(c) KBr
(d) NH2OH
Solution:
H2O is polar and is capable of hydrogen bonding. The
Lewis structure of CS2 shows no lone pair on carbon, which indicates
a linear and nonpolar molecule:
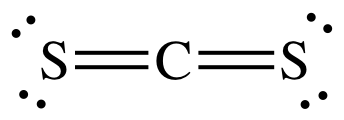
(a) The polar HOCl
will dissolve to a greater extent in the polar water.
(b) The nonpolar
I2 will dissolve to a greater extent in the nonpolar
carbon disulfide.
(c) KBr =
K+ and Br- = ionic, so will dissolve to a greater extent
in the polar water (due to ion-dipole attraction).
(d) NH2OH is capable of
hydrogen bonding, so will dissolve to a greater extent in water, which can
hydrogen bond as well.
Chapter 12 Practice Exercises and Review Quizzes:
12-1) Rank the compounds CaO, K2S, RbI, and SCl2
from lowest to highest melting point and explain.
Click for Solution
12-1) SCl2 = molecular =
lowest melting point
Other three compounds are ionic, so
larger sum of one cation's charge magnitude + one anion's charge magnitude = higher melting point:
CaO = Ca2+
and O2-, sum of charge magnitudes = 2 + 2 = 4
K2S = K+ and
S2-, sum of charge magnitudes = 1 + 2 = 3
RbI = Rb+ and I-, sum of charge magnitudes
= 1 + 1 = 2
Therefore, the order of melting
points = SCl2 < RbI < K2S
< CaO.
12-2) State whether each of the
following is a good or poor conductor of electricity in the solid state and explain:
(a) C(diamond)
(b) K
(c) MgBr2
(d) N2
Click for Solution
12-2)
(a) network
covalent with localized electrons = poor conductor in solid state
(b) potassium
= metallic = good conductor in solid state because electrons are delocalized (and also good conductor in liquid state because electrons are delocalized)
(c) Mg2+ and Br-
= ionic = poor conductor in solid state because cations and anions are immobile (but good conductor in liquid state or
aqueous state because cations and anions are mobile)
(d) nonmetal
= molecular = poor conductor in solid state because electrons are localized (and also poor conductor in liquid state because electrons are localized)
12-3) Rank each of the following
groups from highest to lowest boiling point and explain:
(a) CO2, CH3OH,
CH3OCH3, LiF
(b) Al, Br2, O2,
ICl
Click for Solution
12-3)
(a) LiF =
Li+ and F- = ionic = higher boiling point than the other
three, which are all molecular substances.
CH3OH will have the second highest boiling
point because it is capable of hydrogen bonding. Note that CH3OCH3 is not capable of
hydrogen bonding because H is not bonded directly to O.
The total number of electrons in CH3OCH3
(2 x 6 + 6 x 1 + 8 = 26) and CO2 (6 + 2 x 8 = 22) are similar, so these two
are expected to have roughly equal London forces. However, CH3OCH3 is polar
and, therefore, is also held together by dipole-dipole forces that give it the
third highest boiling point. We
have determined that CH3OCH3 is polar
from the Lewis structure, which shows lone pairs on the oxygen atom that lead
to a bent shape in that region:
On the other hand, CO2
is nonpolar and, therefore, lacks the extra
dipole-dipole attraction, so CO2 will have the lowest boiling point
in the group. We have determined
that CO2 is nonpolar from the Lewis
structure, which shows no lone pairs on center carbon atom, indicating a linear
shape for the molecule:
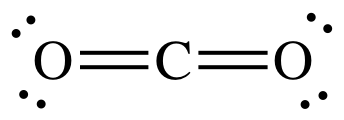
Therefore, the order of boiling
points = LiF > CH3OH > CH3OCH3
> CO2.
(b) Al = metallic = higher boiling
point than the other three, which are all molecular substances.
The total number of electrons in Br2
(2 x 35 = 70) and ICl (53 + 17 = 70) are the same, so these two are expected to have roughly equal London forces. However, ICl
is polar and, therefore, has dipole-dipole forces to
raise the boiling point higher than that of the nonpolar
Br2, which lacks the extra dipole-dipole attractions. The nonpolar
O2 has significantly fewer total electrons (2 x 8 = 16) than Br2,
so the London forces in O2 will be weaker than those in Br2
and, therefore, O2 will have the lowest boiling point in the group.
Therefore, the order of boiling
points = Al > ICl > Br2 > O2.
12-4) Predict whether each solute
below will dissolve to a greater extent in water or benzene, C6H6, and explain:
(a) C10H8
(b) H2CO
(c) NH3
(d) SrI2
Click for Solution
12-4) Water is polar and is capable of hydrogen bonding. Benzene is a hydrocarbon and,
therefore, is considered nonpolar.
(a) The nonpolar
hydrocarbon C10H8 will dissolve to a greater extent in
the nonpolar benzene.
(b) The polar H2CO
will dissolve to a greater extent in the polar water.
(c) NH3 is capable of hydrogen bonding, so will
dissolve to a greater extent in water, which can hydrogen bond as well.
(d) SrI2 = Sr2+
and I- = ionic, so will dissolve to a greater extent in the polar
water (due to ion-dipole attraction).
