Chapter 5: Gases
Section 5-1: The Ideal Gas Law
Section 5-2: Gas Stoichiometry
Section 5-3: Determining Molar Mass of a Gas and Gas Density
Section 5-5: Measuring Pressure with Barometers and Manometers
Section 5-6: Collecting Gases Over Water
Section 5-7: Experiment - Determining the Gas Constant R
Chapter 5 Practice Exercises and Review Quizzes
Section 5-1: The Ideal Gas Law
The following variables will be
used to represent different gas properties:
|
Variable |
Gas Property |
|
n |
moles |
|
P |
pressure |
|
T |
temperature |
|
V |
volume |
The four gas properties above are
related through an equation known as the Ideal
Gas Law:
PV = nRT
When using the Ideal Gas Law with
the gas constant R = 0.0821 L•atm/mol•K, we must
ensure the following:
1. The pressure must be in the unit
atmospheres (atm). Since some lab equipment will give the pressure in the unit
millimeters of mercury (mmHg) or the equivalent torr,
you may need the following conversion:
1 atm = 760 mmHg = 760 torr
2. The temperature must be in the
unit kelvin (K). Since some lab thermometers will give the temperature in
degrees Celsius (°C), you may need the following conversion:
T(K) = T(°C) + 273
Note that the temperature
conversion above involves addition, so the number of sig. fig.s
may change. For example, a
temperature of 22°C with 2 sig. fig.s will be the
equivalent of 22 + 273 = 295 K and, thus, the temperature is considered to have
3 sig. fig.s during calculations that may follow.
3. The volume must be in the unit
liters.
If we know any 3 of the 4 variables
above, we can solve the Ideal Gas Law for the unknown variable, as demonstrated
in the following problems:
Sample Exercise 5A:
(a) A gas at Standard Temperature and Pressure (STP) has a temperature of 273 K
and a pressure of 1.00 atm. What
is the volume of 1.00 mole of gas at STP?
(b) A 0.120 g sample of CH4
gas occupies a volume of 200. mL
at 35°C. What is the pressure of
the gas in mmHg?
Solution:
(a)
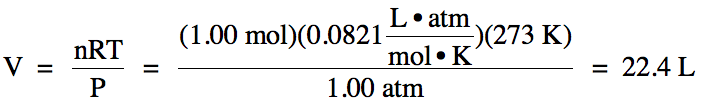
(b)
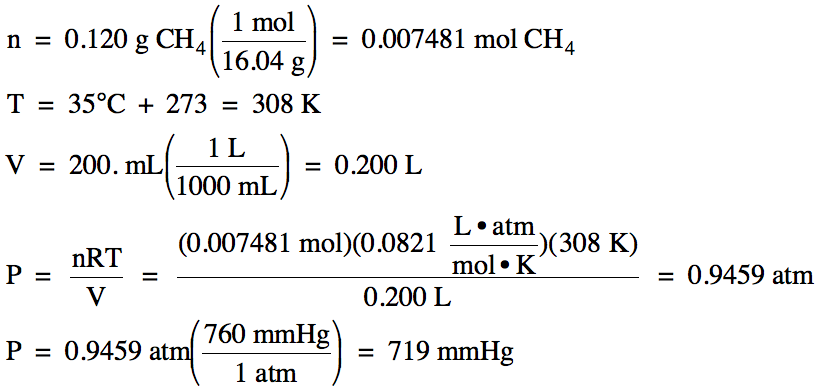
Section 5-2: Gas Stoichiometry
For reactions involving at least
one gas, we can use the Ideal Gas Law to convert to and/or from moles in stoichiometry problems, as demonstrated below:
Sample Exercise 5B:
Given the unbalanced equation
below, what mass of solid sodium must react to produce 845 mL
of hydrogen gas, measured at 21°C and 707 torr?
Na(s) + H2O
(l) → NaOH (aq) + H2 (g)
Solution:
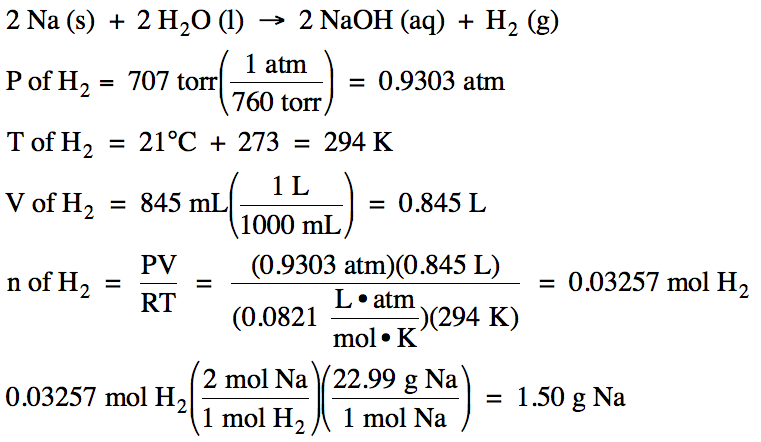
For reactions involving at least
one gas, the Ideal Gas Law can also be used to convert to and/or from moles in
limiting reagent and percent yield problems.
Section 5-3: Determining Molar Mass of a Gas and Gas
Density
If we measure the pressure,
temperature, and volume of an unknown gas, we can solve the Ideal Gas Law for
moles. If we also know the mass of
the gas, we can divide the mass by the moles to determine the molar mass of the
unknown gas:
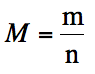
If we have determined the empirical
formula of the unknown gas, we can use the molar mass we have calculated to
determine the molecular formula of the unknown gas, as demonstrated in the
problem below:
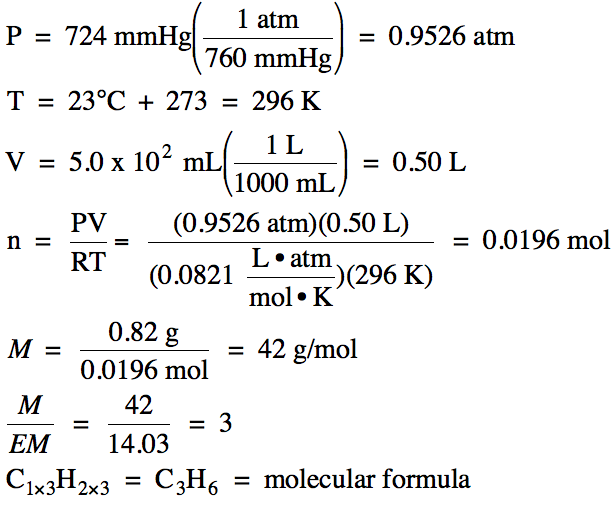
Sample Exercise 5C:
An unknown gas has the empirical
formula CH2. A 0.82
gram sample of the gas was found to occupy 5.0 x 102 mL at 23°C and 724 mmHg. Determine the molar mass and molecular formula of the
unknown gas.
Solution:
We can use the Ideal Gas Law to
substitute for moles in the molar mass equation above:
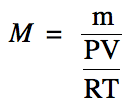
We can then solve this equation for
mass divided by volume to derive a new equation for gas density:
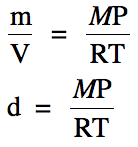
Note that we must use the same
units for P and T in the gas density equation that we used in the Ideal Gas Law
and also that the unit of the calculated density will be g/L, as demonstrated
in the following problem:
Sample Exercise 5D:
What is the density of H2S
gas at 45°C and 787 torr?
Solution:
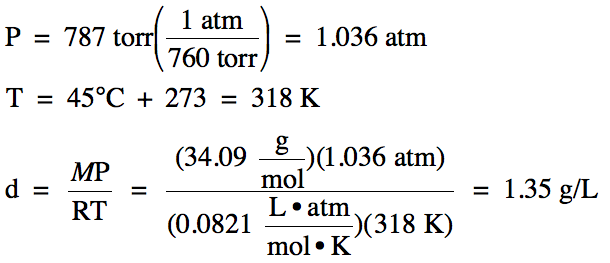
Section 5-4: Other Gas Laws
When two or more properties of a
gas change from a known initial (i) set of values and all but one of the final (f) values are known, the General Gas Law can be used to
calculate the unknown final value:
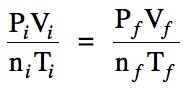
When using the General Gas Law,
note the following:
1. The pressure must be in the same
unit on both sides of the equation but does not necessarily need to be changed
to atm and can remain in mmHg or torr.
2. The volume must be in the same
unit on both sides of the equation but does not necessarily need to be changed
to L and can remain in mL.
3. The temperature must be in K on both sides of the equation!!!
4. Any of the above properties that
are not mentioned can be assumed to be unchanged and, thus, can be eliminated
from both sides of the equation, as demonstrated in the following problem:
Sample Exercise 5E:
A sample of gas occupies a volume
of 245 mL at 25°C and 685 mmHg. What will be the new volume of the gas
if the temperature is increased to 75°C and the pressure in increased to 890. mmHg?
Solution:
Since moles are not mentioned, we
can assume n is unchanged and can be eliminated from the General Gas Law, after
which we can solve for final volume:
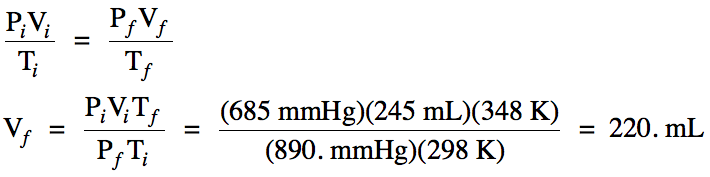
In cases where moles and
temperature are unchanged, Boyle's Law
essentially states that the pressure and volume of a gas are inversely
proportional, which can be represented mathematically by the following
equation:
![]()
In cases where moles and pressure
are unchanged, Charles's Law essentially
states that the volume and temperature in
K of a gas are directly proportional, which can be represented
mathematically by the following equation:
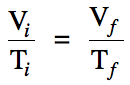
In a mixture of three different
gases A, B, and C, the partial pressure of gas A can be represented as PA,
the partial pressure of gas B can be represented as PB, and the
partial pressure of gas C can be represented as PC. Dalton’s Law of partial pressures
essentially states that the total pressure of a gas mixture is simply the sum
of the partial pressures of each gaseous component:
Ptotal = PA + PB +
PC
The ratio of the partial pressure
of A to the total pressure of the mixture is equal to the mole fraction of A,
which is the ratio of the moles of A to the total moles of gas in the mixture:
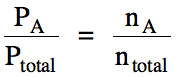
Therefore, the partial pressure of
A can be calculated by multiplying the mole fraction of A by the total pressure
of the mixture:
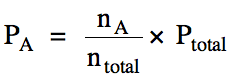
Sample Exercise 5F:
A gaseous mixture of 0.24 mol
helium, 0.12 mol neon, and 0.36 mol argon has a total pressure of 0.96
atm. Calculate the partial
pressure of each gas in the mixture.
Solution:

Section 5-5: Measuring Pressure with Barometers and
Manometers
The pressure exerted by the
collection of gases in the atmosphere on the surface below can be measured
using a barometer and is known as the barometric pressure, Pbar. A typical barometer consists of an
evacuated tube with one closed end and one open end that is inserted into a
container of liquid mercury, as shown below:
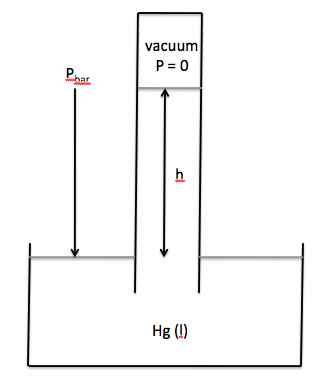
The barometric pressure will be
equal to the height to which the mercury is forced up the tube:
Pbar = h in mmHg
Note that other liquids can be used
instead of mercury in a barometer, but the height in the tube will be inversely
proportional to the density of the liquid. In other words, a liquid with a lower density will rise to a
greater height. To convert a
height in millimeters of one liquid to a height in millimeters of mercury, use the following equation:
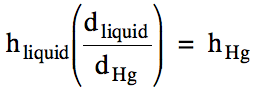
Sample Exercise 5G:
When a barometer containing liquid
benzene was used to measure the barometric pressure, the height in the tube was
found to be 11.0 meters. Given
that the density of liquid benzene is 0.877 g/mL and
the density of liquid mercury is 13.6 g/mL, calculate
the barometric pressure in atm.
Solution:

The pressure of a gas sample can be
measured in the laboratory using a manometer. A typical open-end manometer consists of a curved tube
containing liquid mercury that is attached to the gas sample on one end and
open to the atmosphere on the other end. If the mercury level in the arm attached to the gas is the same and the
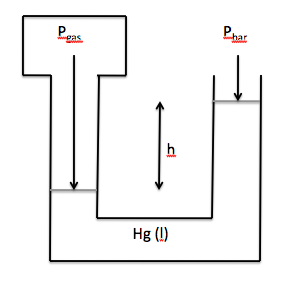
mercury level in the arm open to the atmosphere, then Pgas = Pbar. However, if the mercury level in the arm
attached to the gas is lower than the mercury level in the arm open to the
atmosphere, then Pgas = Pbar + h, where h is the difference in heights
between the mercury levels in the two arms:
If the mercury level in the arm
attached to the gas is higher than the mercury level in the arm open to the atmosphere,
then Pgas = Pbar - h:
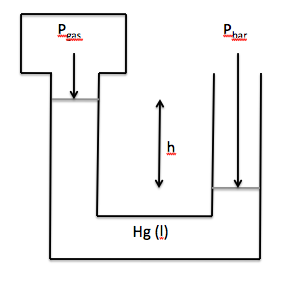
Note that Pbar and h must be in the same unit before h is
added to or subtracted from Pbar:
Sample Exercise 5H:
On a day when the barometric
pressure was 0.967 atm, the pressure of a gas sample
was measured using an open-end manometer. If the mercury level in the arm attached to the gas was 3.1 centimeters
lower than the mercury level in the arm open to the atmosphere, calculate the
pressure of the gas in torr.
Solution:
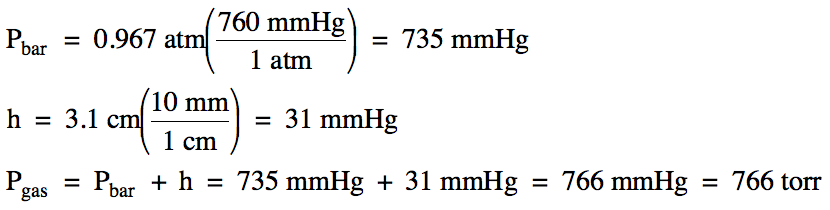
Section 5-6: Collecting Gases Over Water
If we wish to collect the gas
produced in a chemical reaction without any escaping, one strategy is to ensure
that all the gas is released under water where it will rapidly rise straight up
because of the much lower density of the gas relative to the density of
water. If a eudiometer
(gas-measuring tube with volume markings that is closed on one end and open on
the other) is completely filled with water and then inverted into a beaker of water,
gas from a chemical reaction that is released inside the opening of the tube
will rise straight up and displace water from the tube. Thus, the gas produced will now be
trapped in the tube. The tube can
usually be adjusted so that the water levels inside and outside the tube are
the same, as shown below:
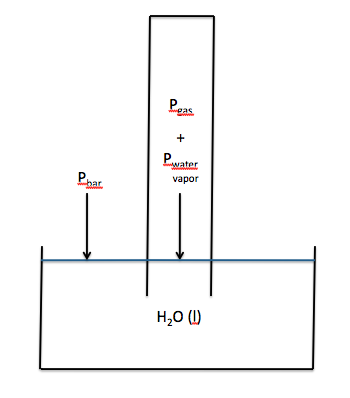
The volume of gas collected can be
determined by reading the volume markings on the eudiometer. Since the gas produced must travel
through the water before being collected over water, we can use a thermometer to
measure the temperature of the water and assume that the temperature of the gas
is the same. Note that a gas
collected over water will be mixed with water vapor. As such, if the water levels inside and outside the tube are
the same, then the following will be true:
Pbar = Pgas + Pwater vapor
The water vapor pressure, which
will vary at different temperatures and can typically be found on a data table,
can be subtracted from the barometric pressure to obtain the partial pressure
of the gas:
Pgas = Pbar - Pwater vapor
Once we know the pressure, volume,
and temperature of the gas, we can calculate the moles of gas produced in the
chemical reaction, after which stoichiometry can be
used to find the mass of one of the reactants used:
Sample Exercise 5I:
On a day when the barometric
pressure was 749 mmHg, oxygen gas was produced in the following unbalanced
decomposition reaction:
KClO3 (s) → KCl (s) + O2 (g)
The oxygen gas was collected over
water at 25°C (water vapor pressure at 25°C = 24 mmHg). If 264 mL of
oxygen gas was collected, calculate the mass of KClO3 decomposed in
the reaction.
Solution:
We first subtract to find the
partial pressure of the oxygen gas and then use the Ideal Gas Law to find the
moles of oxygen gas produced, after which we use the stoichiometry of the balanced equation to determine the mass of KClO3 decomposed:
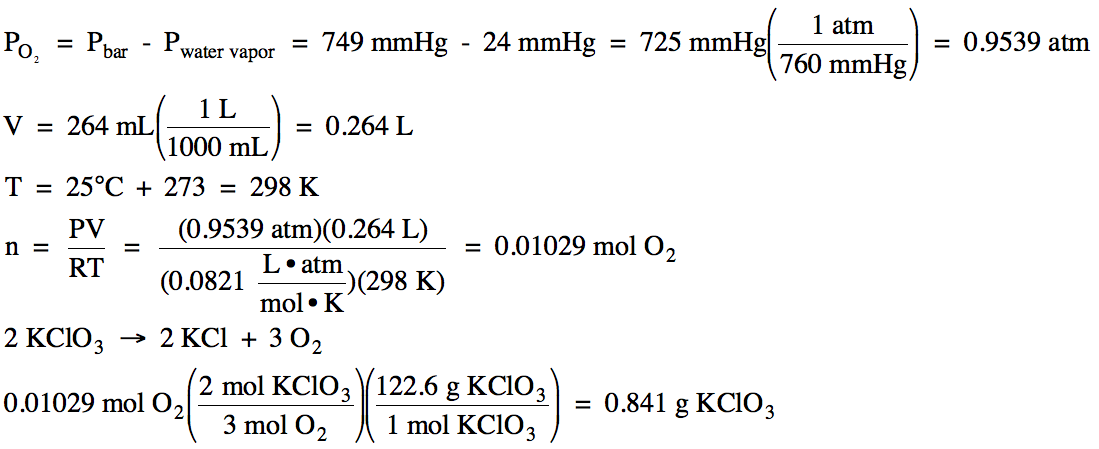
Section 5-7: Experiment – Determining the Gas
Constant R
The value of the gas constant R can
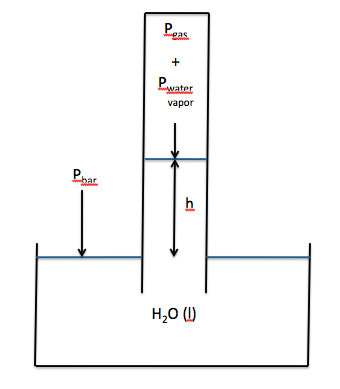
be determined experimentally after collecting a gas over water. In some cases, it may not be possible
to adjust the tube in order to equalize the water levels inside and
outside. As such, the water level
inside the tube may be higher than the water level outside, as shown below:
If the water level inside the tube
is higher than the water level outside, then the following will be true:
Pbar = Pgas + Pwater vapor + h
Therefore, the partial pressure of
the gas can be calculated as follows:
Pgas = Pbar - Pwater vapor - h
Note that h must be converted from
millimeters of water to millimeters of mercury before being subtracted:
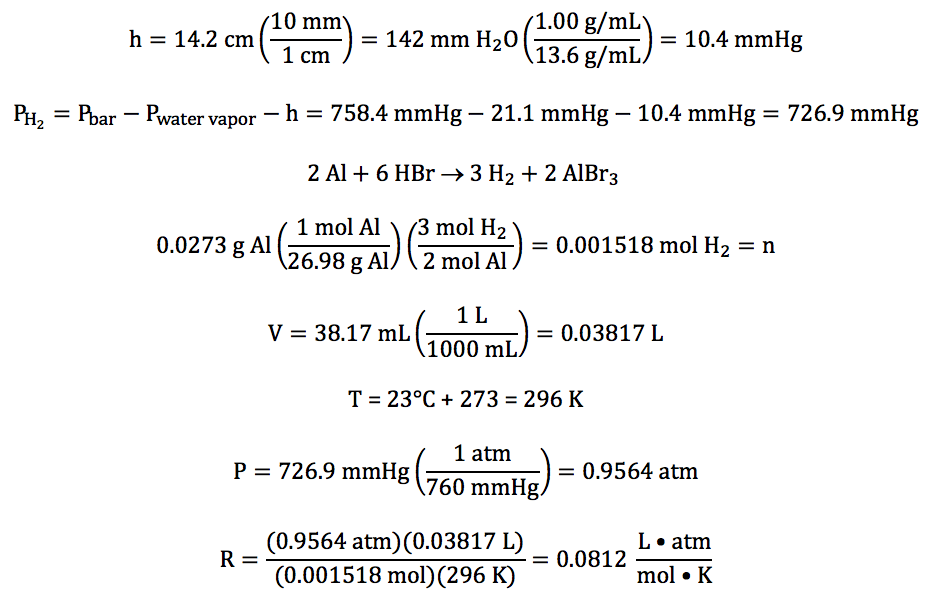
Sample Exercise 5J:
In an experiment to determine the
value of the gas constant R, hydrogen gas was collected over water after being
produced in the following unbalanced reaction:
Al (s) + HBr (aq) → H2 (g) + AlBr3 (aq)
The water level inside the tube was
higher than the water level outside, and the following data was recorded:
mass of aluminum reacted
|
0.0273 g
|
volume of gas
collected
|
38.17 mL
|
temperature of water
|
23°C
|
water vapor pressure at 23°C
|
21.1 mmHg
|
barometric pressure
|
758.4
mmHg
|
difference in water levels (h)
|
14.2 cm H2O
|
Given that the density of water is 1.00 g/mL and the density of mercury is 13.6
g/mL, calculate the experimentally determined value
of R with units to the correct number of significant figures.
Solution:
First, we must convert h to mmHg
before subtracting to obtain the partial pressure of the hydrogen gas. We then can use the mass of aluminum
reacted and the stoichiometry of the balanced
equation to calculate the moles of hydrogen gas, after which we can use the
Ideal Gas Law to calculate the experimentally determined value of R:
Chapter 5 Practice Exercises and Review Quizzes:
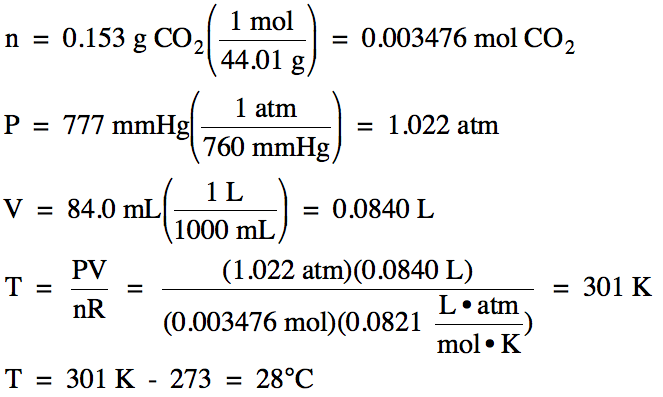
5-1) A
0.153 g sample of carbon dioxide gas occupies a volume of 84.0 mL at 777 mmHg.
What is the temperature of the carbon dioxide in °C?
Click for Solution
5-1)
5-2) Given the unbalanced equation
below for the decomposition of nitroglycerin, if 6.0 kilograms of solid C3H5(NO3)3
decompose, what volume of nitrogen gas will be produced at STP?
C3H5(NO3)3 (s) → CO2 (g) + H2O
(g) + N2 (g) + O2 (g)
Click for Solution
5-2)
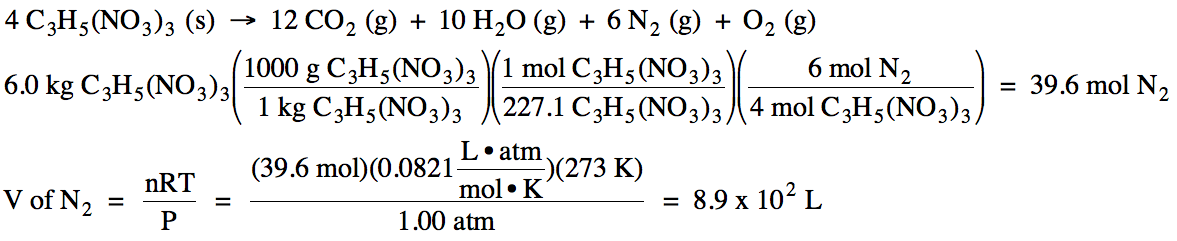
5-3) Given
the unbalanced equation below, if 8.20 grams of solid ZnS
is mixed with 2.93 grams of oxygen gas:
(a) Which is the limiting reagent?
(b) What maximum volume of SO2
gas can form at 26°C and 717 torr?
(c) What mass of the excess reagent
remains when the reaction is complete?
ZnS (s) + O2 (g) → ZnO (s)
+ SO2 (g)
Click for Solution
5-3)
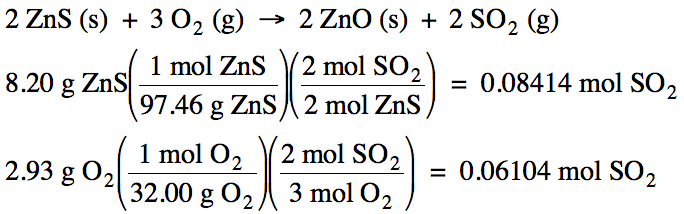
(a) O2 produces less SO2 , so O2 is the limiting reagent.
(b)
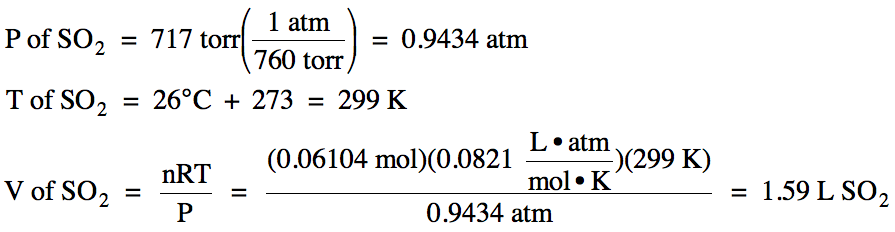
(c)
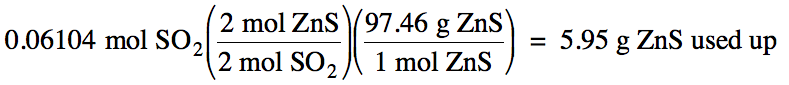
8.20 g
– 5.95 g = 2.25 g ZnS excess
5-4) Given the unbalanced equation
below, if 95 mL of CO gas, measured at 55°C and 794
mmHg, reacts with an excess of solid nickel and then 0.050 grams of Ni(CO)4 is actually collected, what is the
percent yield of the reaction?
Ni (s) + CO
(g) → Ni(CO)4 (g)
Click for Solution
5-4)
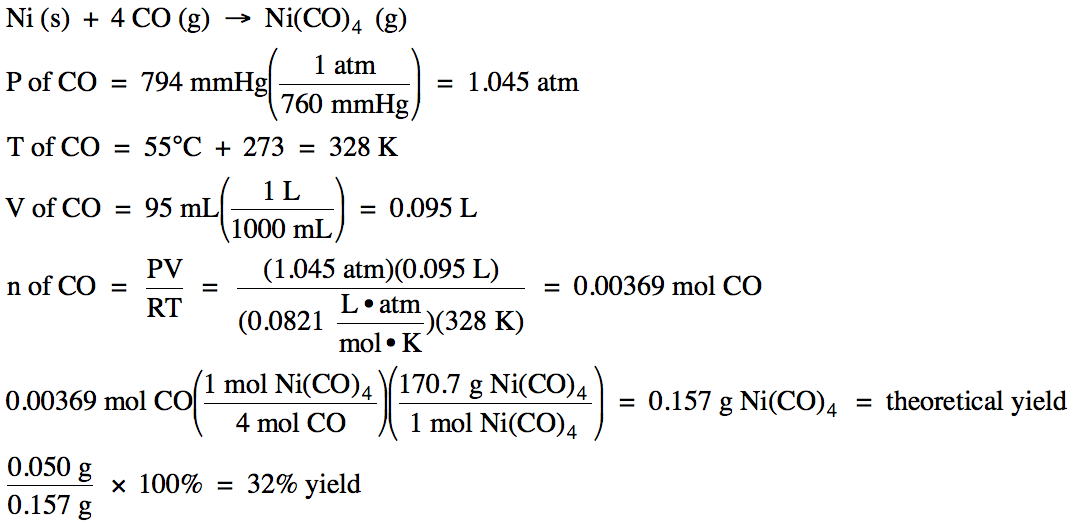
5-5) (a)
An unknown gas was found to be 64.24% carbon and 10.78% hydrogen by mass, with
the remainder being nitrogen.
Determine the empirical formula of the compound.
(b) In a separate experiment, a 10.1 gram sample of the gas
was found to occupy 4.80 L at 525°C and 936 torr. Determine the molar mass and molecular
formula of the unknown gas.
Click for Solution
5-5) (a)
100% - 64.24% C – 10.78% H = 24.98% N by mass
Assume one hundred grams of unknown
compound:
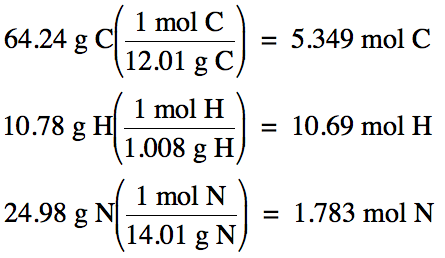
5.349 mol
C: 10.69 mol H: 1.783 mol N (divide each by 1.783)
= 3 mol C:
6 mol H: 1 mol N
Therefore, empirical formula is C3H6N.
(b)
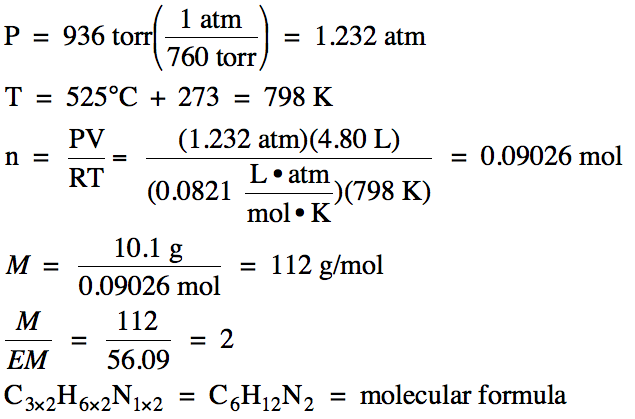
5-6) What
is the density of Cl2 gas at STP?
Click for Solution
5-6)
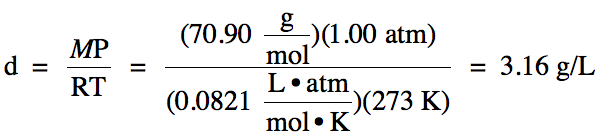
5-7) A sample of gas occupies a
volume of 625 mL at 85°C and 705 torr. What will be the new temperature of the
gas in °C if the volume is decreased to 500. mL and the pressure in increased to 805 torr?
Click for Solution
5-7)
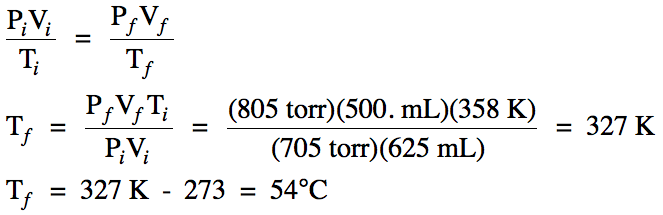
5-8) A gaseous mixture of 0.170 mol methane and 0.510 mol
ethane has a total pressure of 748 mmHg. Calculate the partial pressure of each gas in mmHg.
Click for Solution
5-8)
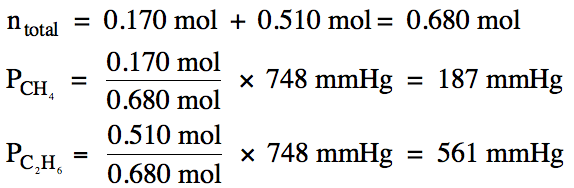
5-9) When a barometer containing liquid glycerol was used to measure the barometric
pressure, the height in the tube was found to be 7.58 meters. Given that the density of liquid
glycerol is 1.26 g/mL and the density of liquid
mercury is 13.6 g/mL, calculate the barometric
pressure in atm.
Click for Solution
5-9)
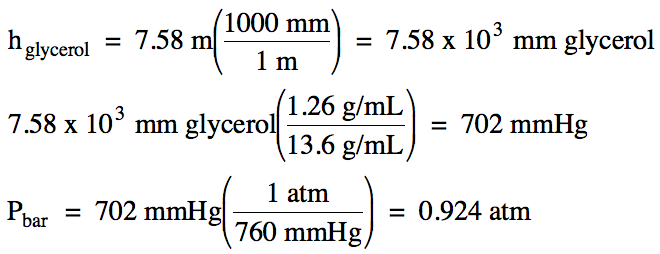
5-10) On a
day when the barometric pressure was 1.01 atm, the
pressure of a gas sample was measured using an open-end manometer. If the mercury level in the arm
attached to the gas was 2.4 centimeters higher than the mercury level in the
arm open to the atmosphere, calculate the pressure of the gas in torr.
Click for Solution
5-10)
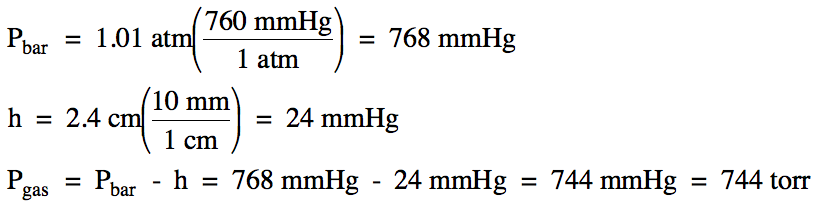
5-11) On a
day when the barometric pressure was 769 mmHg, hydrogen gas was produced in the
following unbalanced reaction:
K (s) + H2O
(l) → KOH (aq) + H2 (g)
The hydrogen gas was collected over
water at 35°C (water vapor pressure at 35°C = 42 mmHg). If 491 mL of
hydrogen gas was collected, calculate the mass of solid potassium metal that
reacted.
Click for Solution
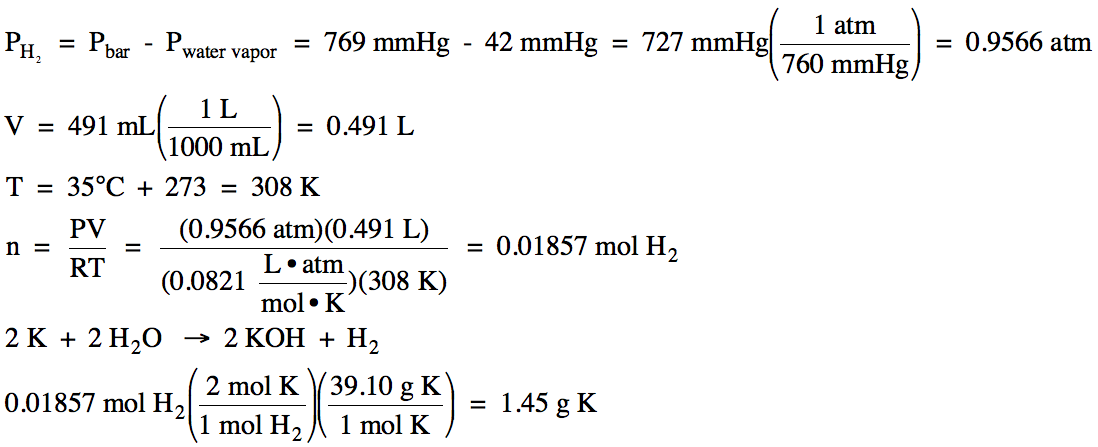
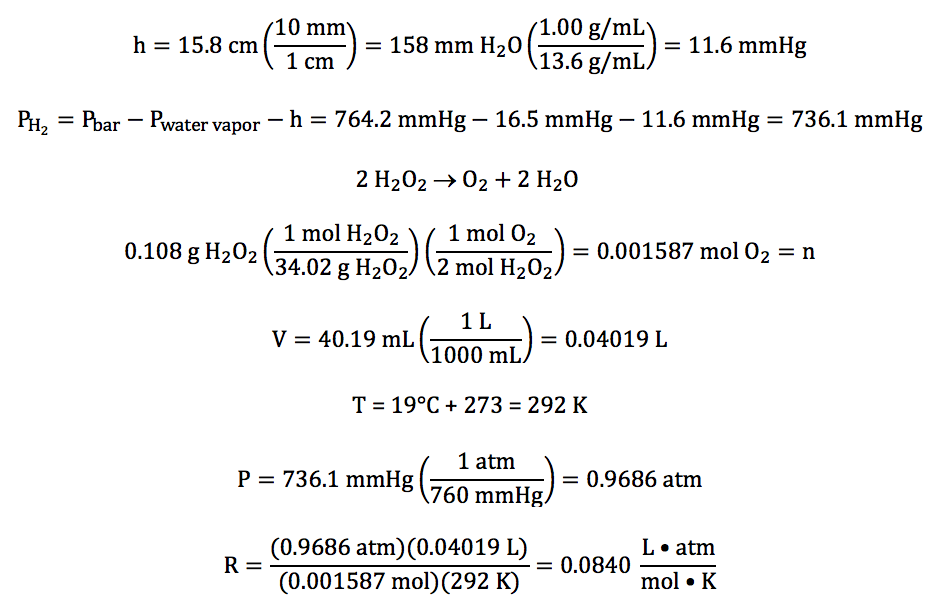
5-12) In an experiment to determine
the value of the gas constant R, oxygen gas was collected over water after
being produced in the following unbalanced reaction:
H2O2 (l) → O2 (g) + H2O (l)
The water level inside the tube was
higher than the water level outside, and the following data was recorded:
mass of H2O2 reacted
|
0.108 g
|
volume of gas
collected
|
40.19 mL
|
temperature of water
|
19°C
|
water vapor pressure at 19°C
|
16.5 mmHg
|
barometric pressure
|
764.2 mmHg
|
difference in water levels (h)
|
15.8 cm H2O
|
Given that the density of water is 1.00 g/mL and the density of mercury is 13.6
g/mL, calculate the experimentally determined value
of R with units to the correct number of significant figures.
Click for Solution