Chapter 18: Qualitative Chemical Kinetics
Section 18-1: Collision Theory and Factors That Increase Chemical Reaction Rates
Section 18-2: Reaction Energy Profiles (Reaction Progress Diagrams)
Section 18-3: Reaction Mechanisms
Chapter 18 Practice Exercises and Review Quizzes
Section 18-1: Collision Theory and Factors That Increase Chemical Reaction Rates
However, for a collision between
reactant molecules to actually result in a reaction, the following requirements
must also be met:
A. The reactant molecules must
collide with the proper orientation or at the proper angle.
B. The total kinetic energy (energy
of motion) of the reactant molecules must be equal to or greater than the
required minimum energy, which is known as the activation energy and will be
different for each different chemical reaction. Therefore, the rates of chemical reactions will generally
increase when the percentage of reactant molecules that
possess the required activation energy is increased by increasing the temperature.
Section 18-2: Reaction Energy Profiles (Reaction
Progress Diagrams)
A reaction energy profile (or
reaction progress diagram) traces the changes in energy that occur as reactants
are transformed into products.
Reactant molecules that collide with the required activation energy are
able to form a higher-energy temporary species known as the transition state
(or activated complex) on the way to becoming product molecules. The activation energy (Ea)
is shown on a reaction energy profile as the difference in energy between the
reactants and the transition state.
The difference in energy between the reactants and products is
represented as ΔH on a reaction energy profile. When the products have a lower energy than the reactants,
the reaction is exothermic and energy is released during the overall reaction:
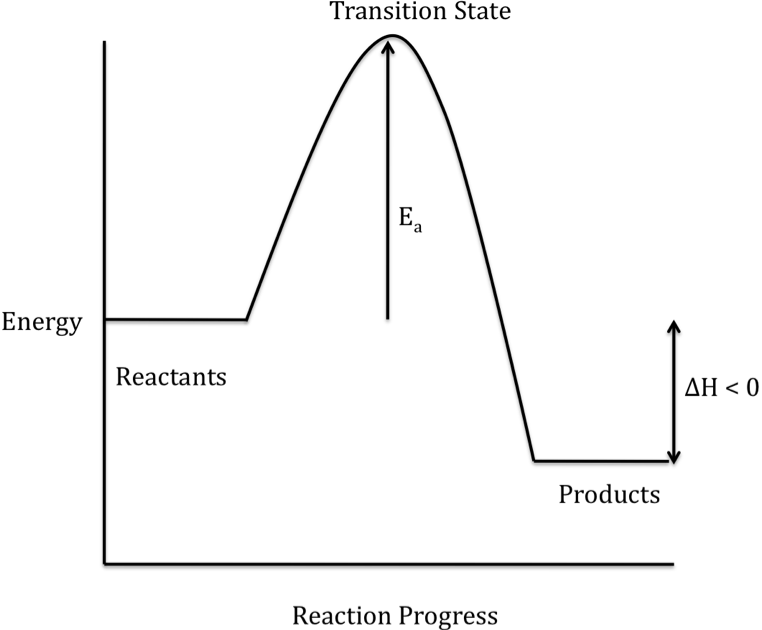
When the products have a higher
energy than the reactants, the reaction is endothermic and energy is absorbed
during the overall reaction:
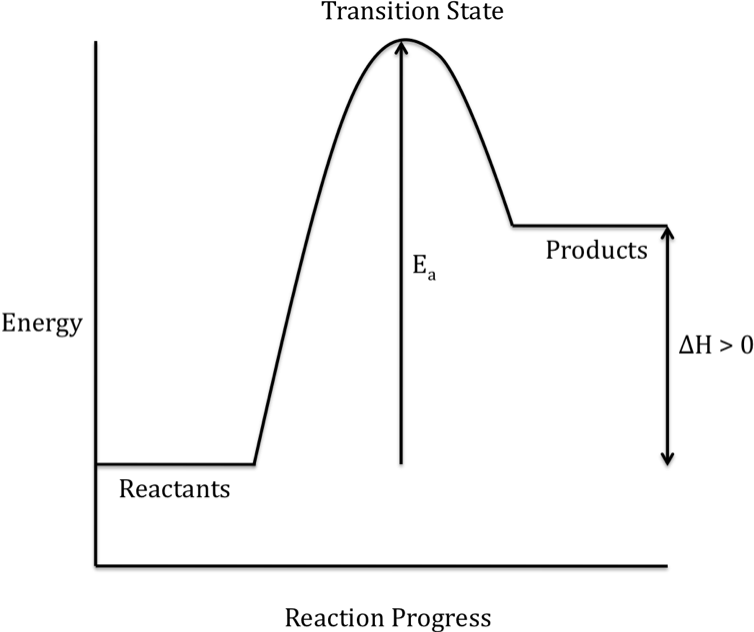
Note that the rate of a chemical
reaction will be higher when the required activation energy is lower, but the
rate of a chemical reaction does not depend on ΔH.
Section 18-3: Reaction Mechanisms
A reaction mechanism depicts at the
molecular level the actual series of elementary steps that occur during a
chemical reaction and add up to give the overall balanced equation. If the hypothetical reaction 2 A + B → C occurred in a single step, three
reactant molecules would need to collide simultaneously. Alternatively, it is possible that the
reaction occurs via a two-step mechanism wherein two A molecules collide in a
first step to form an intermediate (Int.), then the intermediate collides with
a B molecule in a second step to form the product C:
Step
1: A + A → Int.
Step
2: Int. + B → C
Note that the intermediate is
formed in one step but then consumed in the second step, so the intermediate
will not appear in the overall balanced equation:
Sample Exercise 18A:
The following mechanism has been
proposed for a reaction:
Step
1: NO + NO → N2O2
Step
2: N2O2 + O2
→ 2 NO2
Identify the intermediate and write
the overall balanced equation for the reaction.
Solution:
N2O2 is
formed in the first step but then consumed in the second step, so N2O2
is the intermediate that cancels out of the overall balanced equation 2 NO + O2
→ 2 NO2.
Section 18-4: Catalysis
The addition of a catalyst to a
reaction mixture allows the reaction to proceed via an alternate mechanism that
is faster overall than the uncatalyzed
mechanism. For example, if the
hypothetical uncatalyzed reaction A + B → D proceeds via a slow one-step
mechanism, addition of a catalyst (Cat.) can increase the reaction rate by
providing the following faster two-step mechanism:
Step 1: A + Cat. → Int.
Step
2: Int. + B → D + Cat.
Note that the catalyst is consumed
in the first step but then produced in the second step, so the catalyst will
not appear in the overall balanced equation. Also, a relatively small quantity of the catalyst must be
added initially because each catalyst molecule produced in the second step can
be used again as a reactant in the first step.
The alternate mechanism provided by
a catalyst results in a lower-energy transition state and, therefore, a lower
activation energy for the catalyzed reaction, as shown in the reaction energy
profile below for an exothermic reaction (solid curve represents the uncatalyzed reaction):
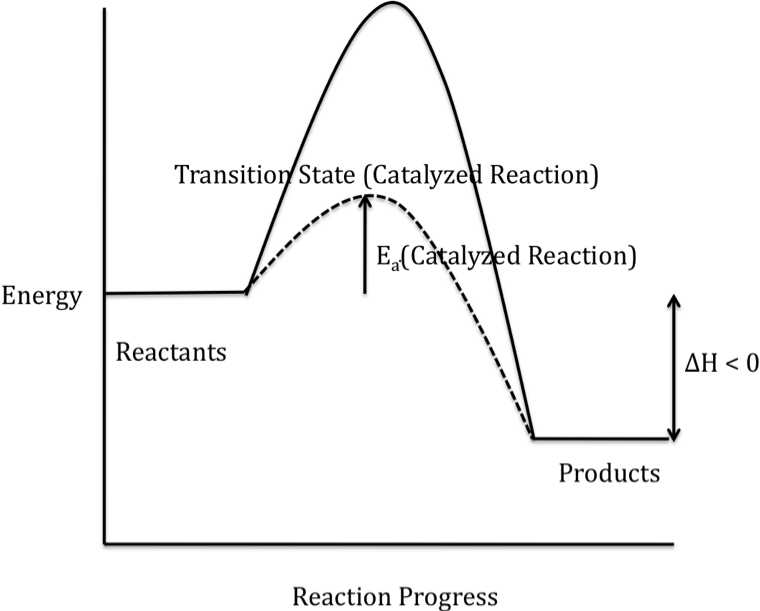
Note that ΔH is unaffected by the
addition of a catalyst because the energies of both the reactants and the
products are unchanged when a catalyst is added.
Chapter 18 Practice Exercises and Review Quizzes:
18-1) Sketch a completely-labeled
reaction energy profile (reaction progress diagram) for an endothermic reaction. Indicate any effects a catalyst would
have on the sketch.
Click for Solution
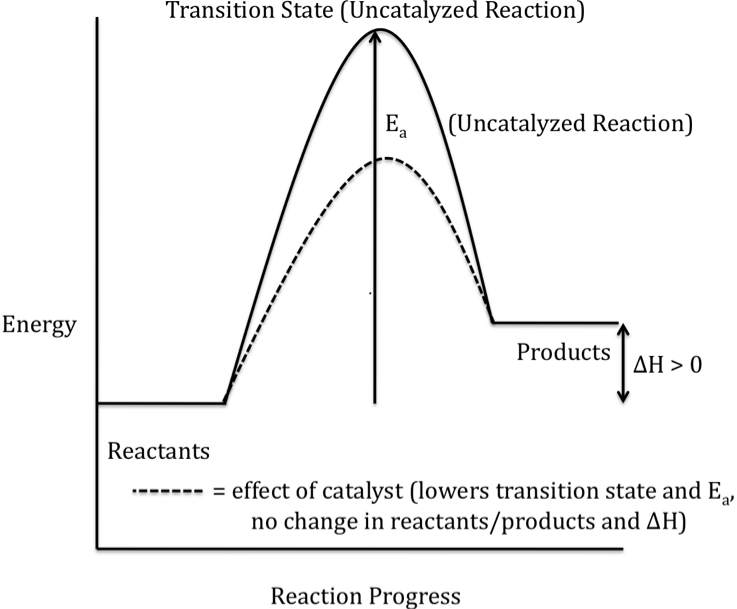
18-2) The
following mechanism has been proposed for a reaction:
Step
1: H2 + ICl → HI + HCl
Step
2: HI + ICl
→ HCl + I2
Identify the intermediate and write
the overall balanced equation for the reaction.
Click for Solution
18-2) HI is formed in the first step but
then consumed in the second step, so HI is the intermediate that cancels out of
the overall balanced equation 2 ICl + H2 → 2 HCl +
I2.
