Chapter 11: Molecular Geometry, Polarity of
Molecules, and Advanced Bonding Theory
Section 11-1: Molecular Geometry: Using VSEPR Theory to Determine Three-Dimensional Shapes and Bond Angles
Section 11-2: Polarity of Molecules
Section 11-4: Sigma and Pi Bonds
Section 11-5: Molecular Orbital (MO) Theory
Chapter 11 Practice Exercises and Review Quizzes
Section 11-1: Molecular Geometry: Using VSEPR Theory to Determine
Three-Dimensional Shapes and Bond Angles
Diatomic molecules and ions are
linear, regardless of the number of covalent bonds between the atoms. For molecules or ions with three or
more bonded atoms, the Lewis structure indicates which specific atoms are bonded
together and the number of bonds between the atoms. However, the Lewis structure does not necessarily provide an
accurate depiction of the three-dimensional shape and bond angles in the
molecule or ion.
Valence-Shell Electron-Pair
Repulsion (VSEPR) Theory suggests
that the valence electron domains comprised of covalent bonds and lone pairs adjacent to the central atom in a
molecule or ion will be arranged in a three-dimensional shape that minimizes
electron repulsion between the bonds and lone pairs. We will use the VSEPR notation ABxEy to categorize molecules and
ions, where A represents the center atom, x represents the number of outer
atoms (B) bonded to the center atom A, and y represents the number of lone
pairs (E) on the center atom A.
Once we know the VSEPR notation for an atom or ion, we can determine the
three-dimensional shape and bond angles.
When we draw three-dimensional sketches of molecules or ions on paper,
we will use solid straight lines to represent bonds oriented in the plane of
the paper, dashed wedges to represent bonds oriented back behind the plane of
the paper, and solid wedges to represent bonds oriented forward out of the
paper. We will also only include
lone pairs on the center atom in sketches, but not lone pairs on the outer atoms as these generally do not affect the shape according
to VSEPR Theory:
I. AB2E0 = AB2 = two
outer atoms bonded to center atom + no lone pairs on center atom = molecule is linear:
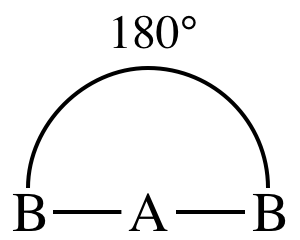
II. AB3E0 = AB3 = three
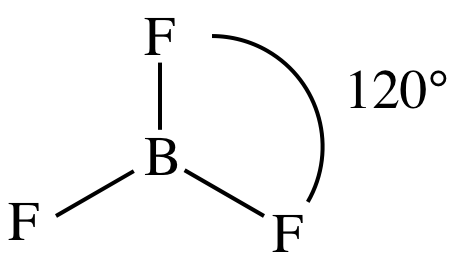
outer atoms bonded to center atom + no lone pairs on center atom = molecule is trigonal planar:
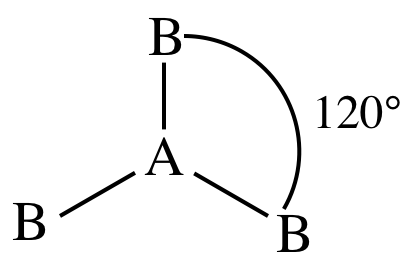
III. AB2E1 = two outer atoms bonded to center
atom + one lone pair on center atom = molecule is bent:
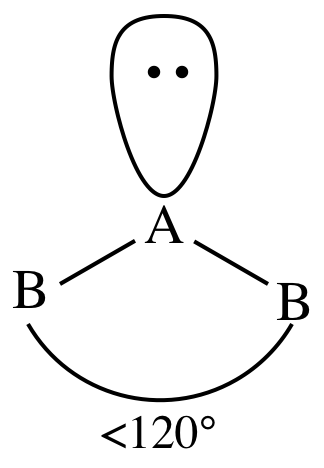
The bond angle is decreased below
120° because the lone pair has a slightly greater repulsive effect than the
electrons in the covalent bonds between A and B.
IV. AB4E0 = AB4 = four
outer atoms bonded to center atom + no lone pairs on center atom = tetrahedral:
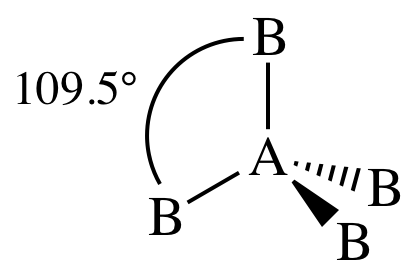
V. AB3E1 = three outer atoms bonded to
center atom + one lone pair on center atom = trigonal pyramidal:

The bond angles are decreased below
109.5° because the lone pair has a slightly greater repulsive effect than the
electrons in the covalent bonds between A and B.
VI. AB2E2 = two outer atoms bonded to center
atom + two lone pairs on center atom = bent:
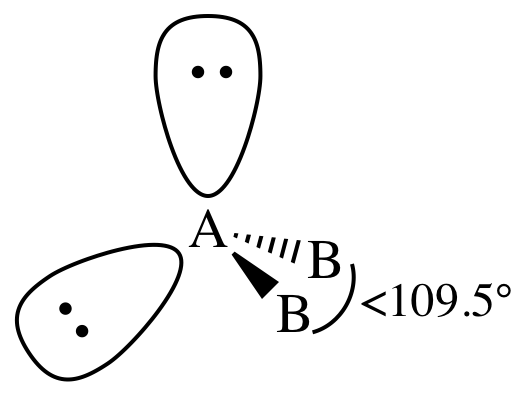
In the AB2E2
= bent case, the extra electron repulsion caused by the second lone pair on the
center atom may cause the B-A-B bond angle to decrease even further below
109.5° than the decrease expected in the AB3E1 = trigonal pyramidal case with only one lone pair on the
center atom.
In contrast to the Lewis
structures, our three-dimensional sketches will neglect the differences between
and, thus, not distinguish among single, double, and triple bonds, as
demonstrated in the following problem:
Sample Exercise 11A:
Draw the Lewis structure, name the
molecular geometry (shape), draw a three-dimensional sketch, and indicate the
bond angle for each of the following molecules and ions:
(a) CO2
(b) NH3
(c) CO32-
(d) CH4
(e) NO2-
(f) H2O
Solution:
(a)
Lewis structure:
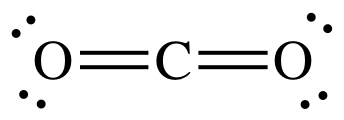
AB2
= linear
3-D sketch:
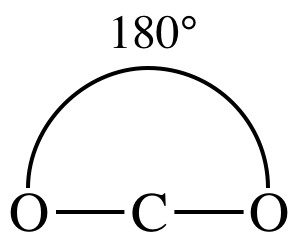
(no need
to distinguish between single v. double v. triple bonds in sketch)
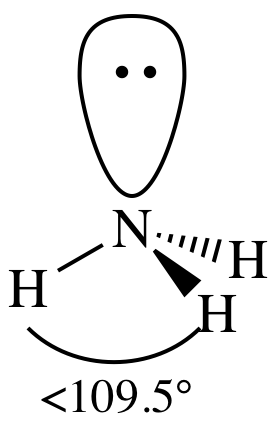
(b)
Lewis structure:
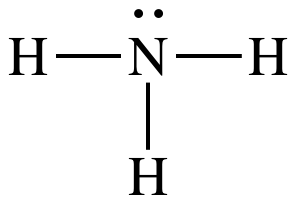
AB3E1
= trigonal pyramidal
3-D sketch:
(c)
Lewis structure:
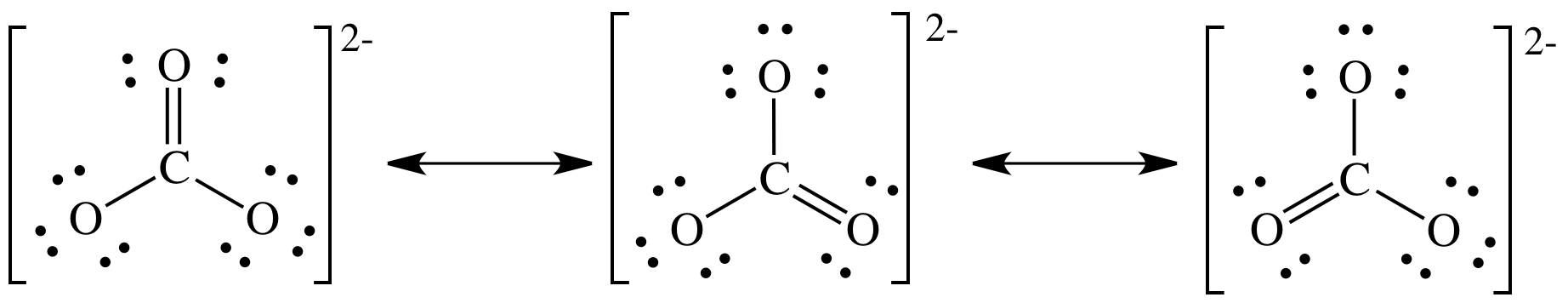
Note that we do not actually need
to draw all the resonance structures to determine the shape as we can see from
any one of the three resonance structures that the carbonate ion = AB3
= trigonal planar.
3-D sketch:
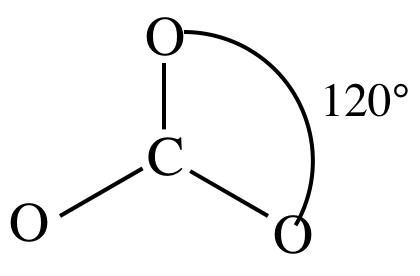
(d)
Lewis structure:
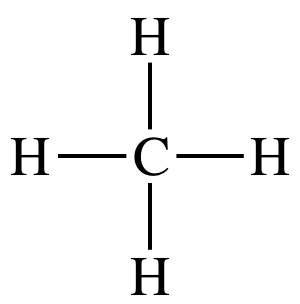
AB4
= tetrahedral
3-D sketch:
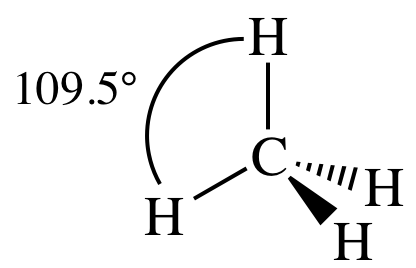
(e)
Lewis structure:
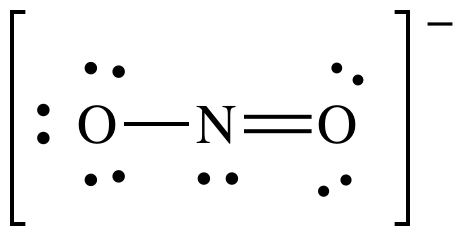
AB2E1
= bent (no need to show all resonance structures to determine shape)
3-D sketch:
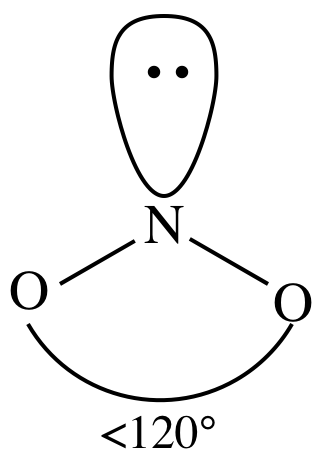
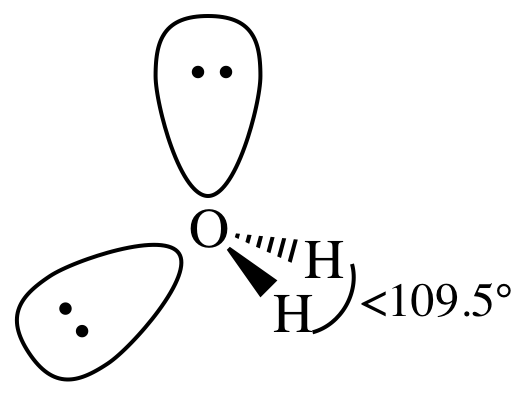
(f)
Lewis structure:
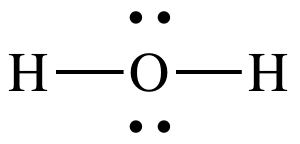
AB2E2
= bent
3-D sketch:
For molecules or ions with more
than one center atom, we can describe the shape in the region of each center
atom as demonstrated in the following problem:
Sample Exercise 11B:
Draw the Lewis structure for acetic
acid. Name the molecular geometry
and indicate the bond angles in the region of each center atom.
Solution:
Acetic acid = CH3COOH. All organic acids with the ending COOH
have a group of atoms (in this case CH3) single-bonded to the carbon
in the COOH. The carbon in the
COOH is double-bonded to one oxygen and single-bonded
to the second oxygen, with the hydrogen in the COOH single-bonded to the second
oxygen. Therefore, the Lewis
structure is:
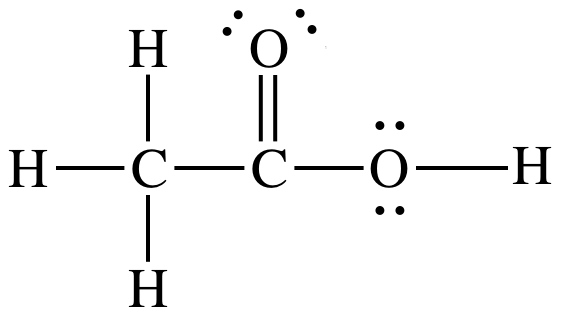
carbon on the left = AB4 = tetrahedral, bond
angles = 109.5°
carbon in center = AB3 = trigonal
planar, bond angles = 120°
oxygen on right = AB2E2 = bent, bond
angle = <109.5°
For molecules or ions with an
“expanded octet” on the center atom, lone pair repulsion will also decrease the bond angle(s), except in the two cases below AB2E3 = linear and AB4E2= square planar:
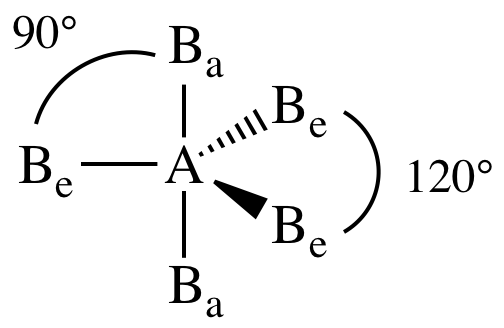
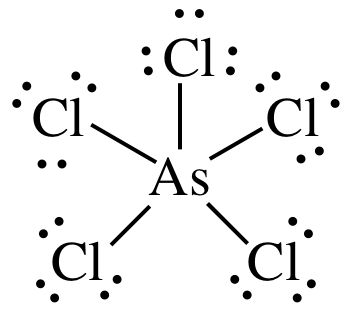
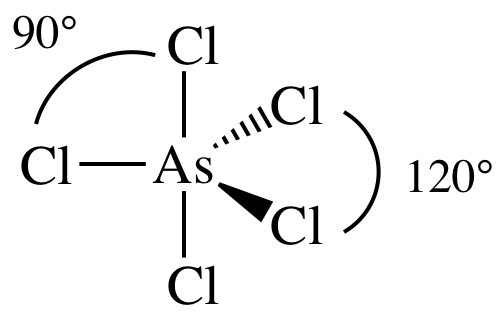
VII. AB5E0 = AB5 = five
outer atoms bonded to center atom + no lone pairs on center atom = trigonal bipyramidal. The two outer atoms occupying an axial
position (Ba) are not equivalent to the three outer atoms occupying
an equatorial position (Be):
VIII. AB4E1 = four outer atoms bonded to
center atom + one lone pair on center atom = seesaw. The lone pair
must occupy an equatorial position to minimize electron repulsion:

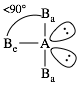
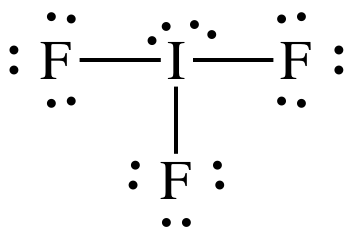
IX. AB3E2 = three outer atoms bonded to
center atom + two lone pairs on center atom = T-shaped. Both lone
pairs must occupy equatorial positions to minimize electron repulsion:
X. AB2E3 = two outer atoms bonded to center
+ three lone pairs on center = linear. All three lone pairs must occupy
equatorial positions to minimize electron repulsion:

XI. AB6E0 = AB6 = six
outer atoms bonded to center + no lone pairs on center = octahedral:
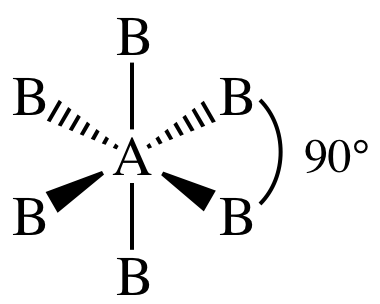
XII. AB5E1 = five outer atoms bonded to
center + one lone pair on center = square
pyramidal:

XIII. AB4E2 = four outer atoms bonded to
center + two lone pairs on center = square
planar:
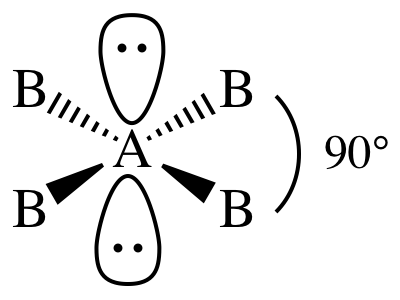
Sample Exercise 11C:
Draw the Lewis structure, name the
molecular geometry (shape), draw a three-dimensional sketch, and indicate the
bond angle(s) for each of the following molecules and ions:
(a) BrCl4-
(b) BrF6+
(c) I3-
(d) IBr4+
(e) PCl5
(f) SF3-
(g) SeCl5-
Solution:
(a)
Lewis
structure:
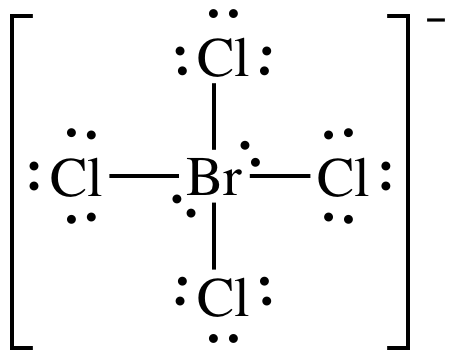
AB4E2 = square planar, 3-D sketch:
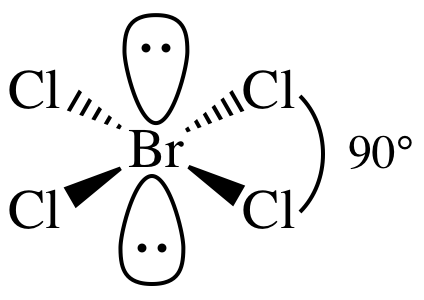
(b)
Lewis
structure:
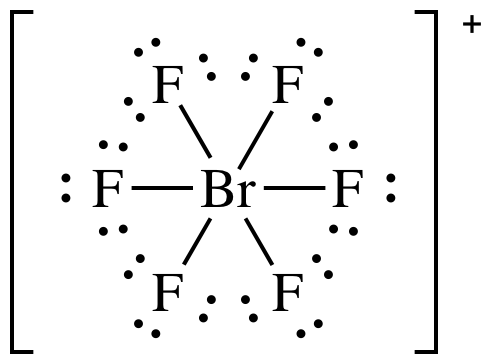
AB6 = octahedral, 3-D sketch:
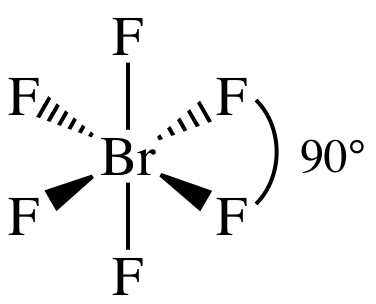
(c)
Lewis
structure:
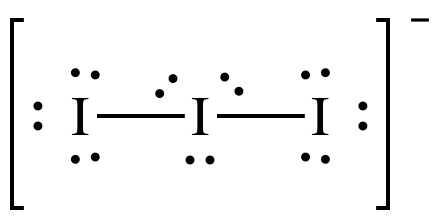
AB2E3 = linear, 3-D sketch:
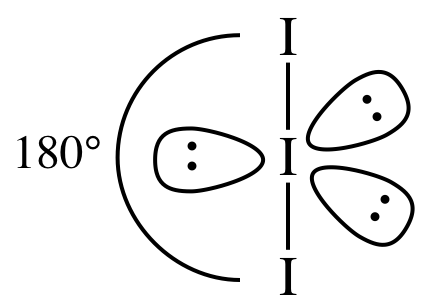
(d)
Lewis
structure:
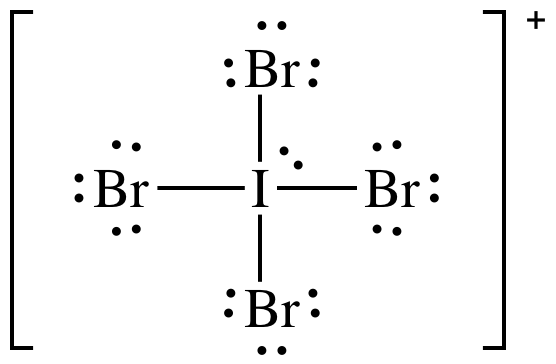
AB4E1 = seesaw, 3-D sketch:

(e)
Lewis
structure:
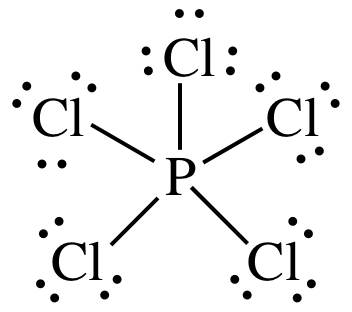
AB5 =
trigonal bipyramidal, 3-D sketch:
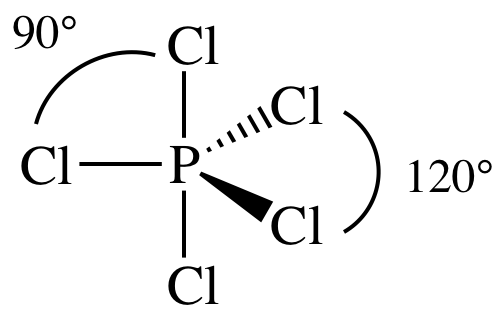
(f)
Lewis
structure:
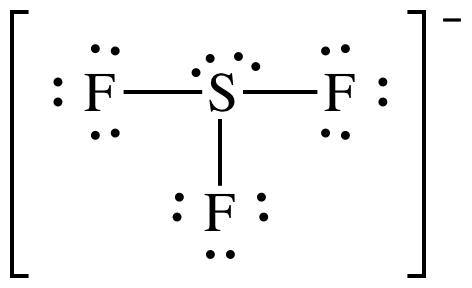
AB3E2 = T-shaped, 3-D sketch:

(g)
Lewis
structure:
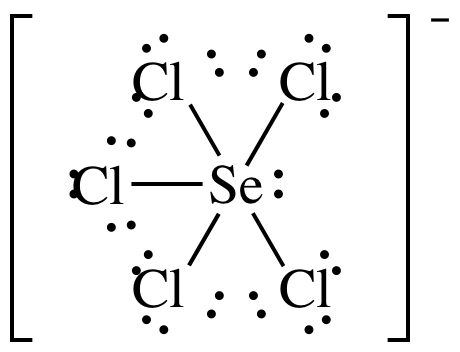
AB5E1 = square pyramidal, 3-D sketch:

Section 11-2: Polarity of Molecules
In a polar covalent bond, the
electrons will be more attracted toward the more electronegative atom. We can indicate the direction in which
the electrons are shifted in a polar covalent bond by placing a bond dipole
arrow parallel to the bond in a sketch of the molecule, with the head of the
arrow closer to the more electronegative atom and the + end of the arrow closer
to the less electronegative atom.
For the molecule BrF, the head of the bond
dipole arrow will be closer to the more electronegative F atom and the + end of
the bond dipole arrow will be closer to the less electronegative Br atom:
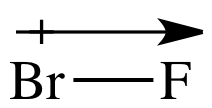
A dipole moment (μ) is
essentially a measurement of the overall net shift of electrons toward a
particular direction in a neutral molecule. A molecule with no identifiable direction toward which the
electrons are shifted is said to be a nonpolar
molecule with zero dipole moment (μ = 0). A molecule with an identifiable
direction toward which the electrons are shifted is said to be a polar molecule
with a dipole moment greater than zero (μ >
0).
In a sketch of the linear carbon
dioxide molecule, the heads of the two bond dipole arrows will be closer to the
more electronegative outer oxygen atoms:
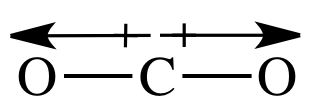
However, since the two bond dipole
arrows essentially cancel each other out because they are equal in magnitude
but are oriented in opposite directions, carbon dioxide is a nonpolar molecule (μ = 0).
In a sketch of the bent water
molecule, the head of the two bond dipole arrows will be closer to the more
electronegative center oxygen atom:
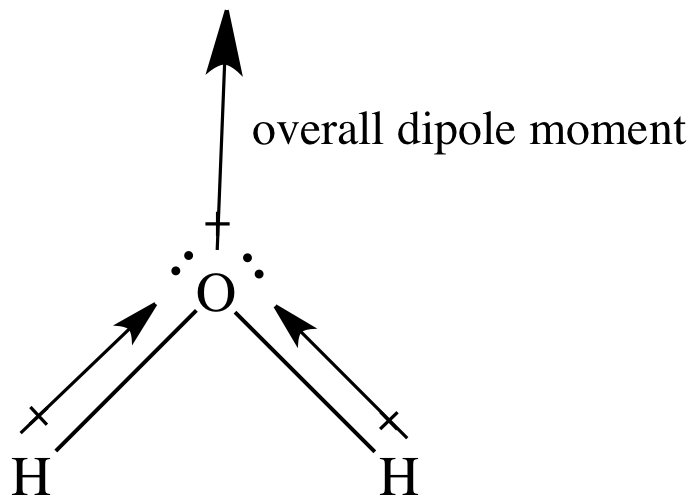
In the case of water, the two bond
dipoles arrows are equal in magnitude but do not cancel each other out. We see that there is an overall net
shift of electrons and, therefore, a dipole moment oriented toward the oxygen
end of the molecule. Thus, water
is a polar molecule (μ >
0).
The following general guideline
indicates which categories of molecules will be nonpolar
and which categories of molecules will be polar:
Molecules with no lone pairs on the center atom will generally be nonpolar if all the outer atoms are the same element. Molecules with one or more lone pairs
on the center atom and molecules with outer atoms that are different elements
will generally be polar.
For each different category, the
table below summarizes the molecular geometry (shape), bond angle, and whether
the molecule is polar or nonpolar:
|
VSEPR Notation |
Name of Molecular Geometry (Shape) |
Bond Angle |
Polar or Nonpolar
Molecule? |
|
AB2 |
linear |
180° |
nonpolar* |
|
AB3 |
trigonal planar |
120° |
nonpolar* |
|
AB2E1 |
bent |
<120° |
polar |
|
AB4 |
tetrahedral |
109.5° |
nonpolar* |
|
AB3E1 |
trigonal pyramidal |
<109.5° |
polar |
|
AB2E2 |
bent |
<109.5° |
polar |
*unless outer atoms are
different elements
Sample Exercise 11D:
Draw the Lewis structure, name the
molecular geometry (shape), draw a three-dimensional sketch, indicate the bond
angle, and state whether each of the following molecules is polar or nonpolar:
(a) BCl3
(b) CH2F2
(c) HCN
(d) SCl2
(e) PF3
(f) HNO
Solution:
(a)
Lewis structure:
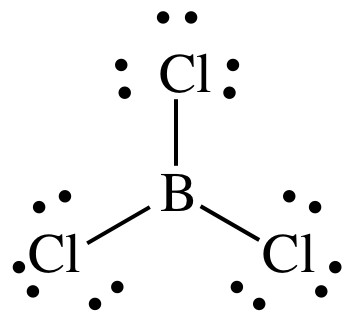
AB3
= trigonal planar
3-D sketch:
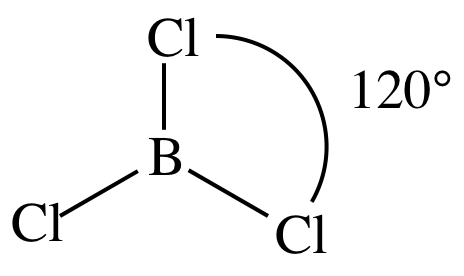
nonpolar molecule
(b)
Lewis structure:
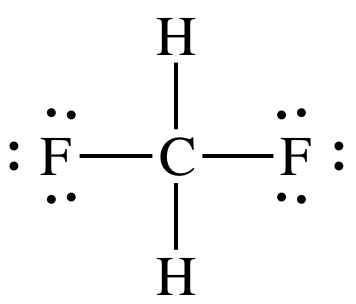
AB4
= tetrahedral
3-D sketch:
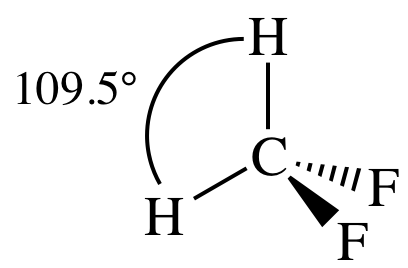
polar molecule (different outer elements)
(c)
Lewis structure:
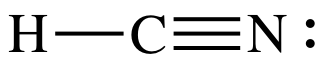
AB2 = linear
3-D sketch:

polar molecule (different outer elements)
(d)
Lewis structure:
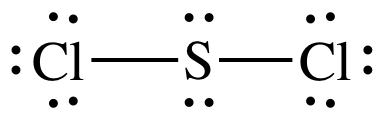
AB2E2
= bent
3-D sketch:
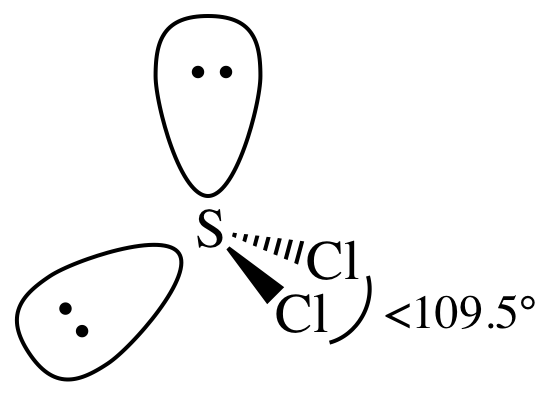
polar molecule
(e)
Lewis structure:
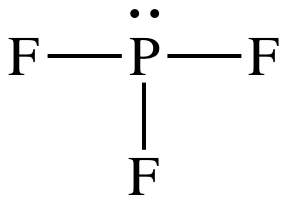
AB3E1
= trigonal pyramidal
3-D sketch:
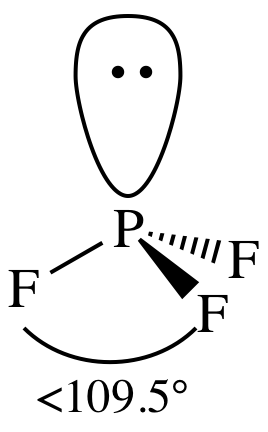
polar molecule
(f)
Lewis structure:
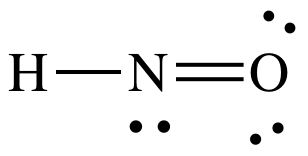
AB2E1
= bent
3-D sketch:
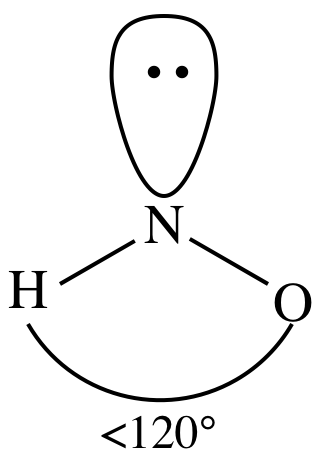
polar molecule
For molecules with an “expanded
octet” on the center atom, we will focus only on those where all the outer
atoms are the same element. Although the general guideline above stating that no lone pairs on
center atom = nonpolar molecule and one or more lone pairs on center atom =
polar molecule still applies in many cases, note that the orientation of the
bonds in AB2E3 = linear and AB4E2 =
square planar molecules results in those molecules being nonpolar.
For each different category of
molecules with “expanded octets”, the table below summarizes the molecular
geometry (shape), ideal bond angle(s), and whether the molecule is polar or nonpolar:
VSEPR Notation
|
Name of Molecular Geometry (Shape)
|
Bond Angle(s)
|
Polar or Nonpolar Molecule?
|
AB5
|
trigonal
bipyramidal
|
90°, 120°
|
nonpolar
|
AB4E1
|
seesaw
|
<90°, <120°
|
polar
|
AB3E2
|
T-shaped
|
<90°
|
polar
|
AB2E3
|
linear
|
180°
|
nonpolar
|
AB6
|
octahedral
|
90°
|
nonpolar
|
AB5E1
|
square
pyramidal
|
<90°
|
polar
|
AB4E2
|
square
planar
|
90°
|
nonpolar
|
Sample Exercise 11E:
Draw the Lewis structure, name the
molecular geometry (shape), draw a three-dimensional sketch, indicate the ideal bond
angle(s), and state whether each of the following molecules is polar or
nonpolar:
(a) AsF5
(b) BrF5
(c) ICl3
(d) KrF4
(e) SF4
(f) SeCl6
(g) XeCl2
Solution:
(a)
Lewis
structure:
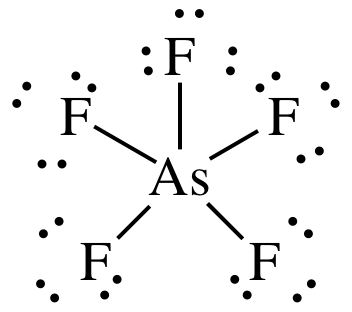
AB5 =
trigonal bipyramidal, 3-D sketch:
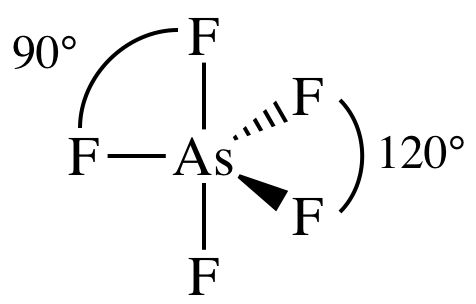
nonpolar
molecule
(b)
Lewis
structure:
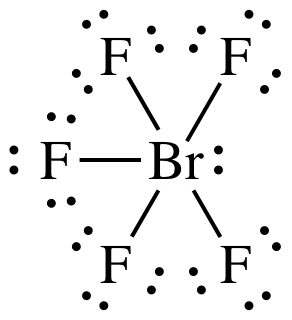
AB5E1 = square pyramidal, 3-D sketch:

polar
molecule
(c)
Lewis
structure:
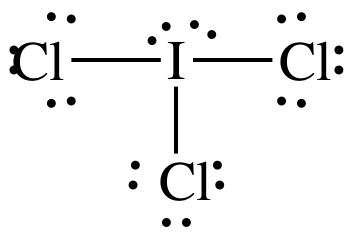
AB3E2 = T-shaped, 3-D sketch:

polar
molecule
(d)
Lewis
structure:
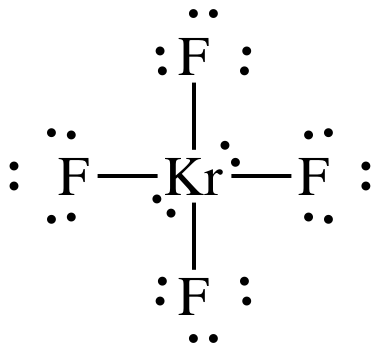
AB4E2 = square planar, 3-D sketch:
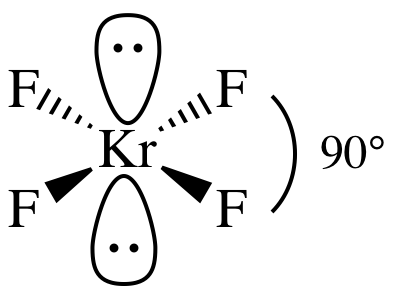
nonpolar
molecule
(e)
Lewis
structure:

AB4E1 = seesaw, 3-D sketch:

polar
molecule
(f)
Lewis
structure:
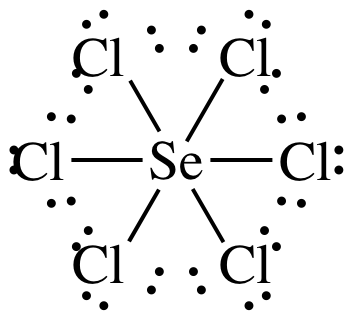
AB6 = octahedral, 3-D sketch:
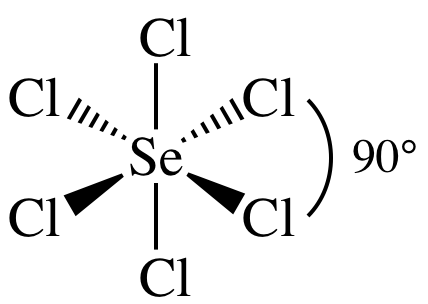
nonpolar
molecule
(g)
Lewis
structure:
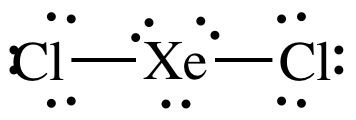
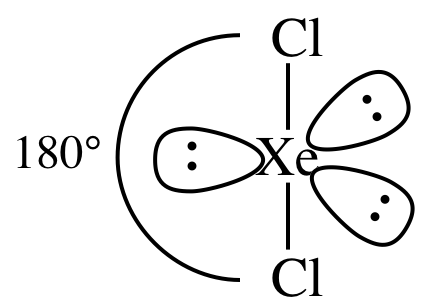
nonpolar
molecule
Section 11-3: Hybridization
Valence bond theory generally
suggests that a covalent bond is formed when an orbital on one atom occupied by
an unpaired valence electron overlaps partially with an orbital on a second
atom occupied by an unpaired valence electron. Increased electron density in the region of overlap between
the atoms attracts the positively-charged nuclei toward each other.
In some cases, valence bond models
depicting overlap between unmodified s and p atomic orbitals would lead to
predictions of bond angles and other properties that do not match experimental
data. Hybridization theory
suggests that the valence s orbital and one or more valence p orbitals from the
center atom undergo a change during the bonding process where the original
atomic orbitals involved are mixed to create an equivalent number of new hybrid
orbitals as follows:
A. If the ideal bond angle in the
molecule or ion being formed is 109.5°, one s orbital and three p orbitals from
the center atom will mix to create four identical sp3 hybrid orbitals arranged tetrahedrally 109.5° away
from each other around the center atom.
B. If the ideal bond angle in the
molecule or ion being formed is 120°, one s orbital and two p orbitals from the
center atom will mix to create three identical sp2 hybrid orbitals arranged in a plane 120° away from
each other around the center atom.
C. If the ideal bond angle in the
molecule or ion being formed is 180°, one s orbital and one p orbital from the
center atom will mix to create two identical sp hybrid orbitals arranged linearly 180° away from each other on
opposite sides of the center atom.
The table below summarizes the
center atom hybridization based on VSEPR notation:
VSEPR Notation
|
Center Atom Hybridization
|
AB2
|
sp
|
AB3
|
sp2
|
AB2E1
|
sp2
|
AB4
|
sp3
|
AB3E1
|
sp3
|
AB2E2
|
sp3
|
Sample Exercise 11F:
Draw the Lewis structure and
indicate the center atom hybridization for each of the following molecules and
ions:
(a) BrNO
(b) ClCN
(c) SF3+
Solution:
(a)
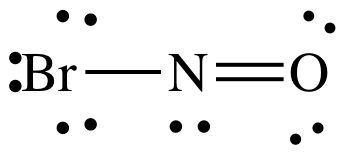
AB2E1 = sp2
(b)
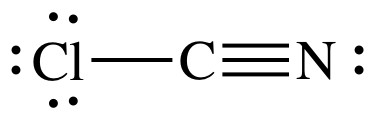
AB2 = sp
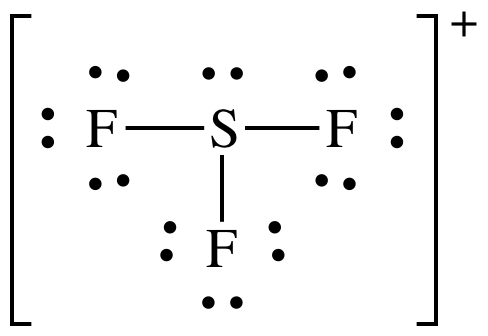
AB3E1 = sp3
Section 11-4: Sigma and Pi Bonds
A bond formed as a result of
end-to-end overlap of orbitals from two neighboring atoms is known as a sigma (σ) bond. Consider the Lewis structure
of C2H4:
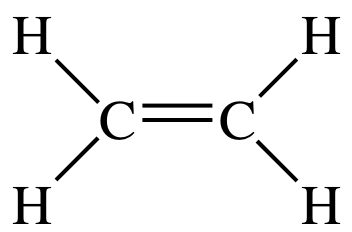
Both carbon atoms in the molecule
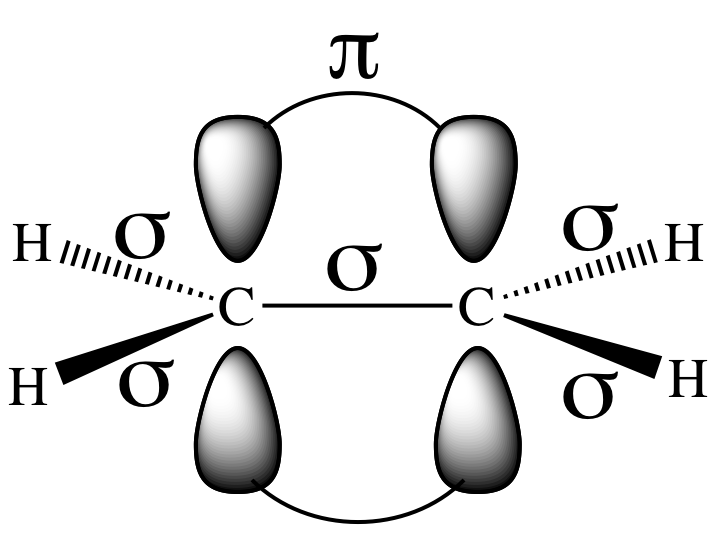
have three sp2 hybrid orbitals (AB3), each of which is
occupied by an unpaired valence electron, that are 120° away from each other in
the same plane. A sigma bond will
be formed between the carbon atoms as a result of end-to-end overlap between
two sp2 orbitals, one from each carbon. In addition, sigma bonds will be formed between each
hydrogen and a carbon as a result of end-to-end overlap between the 1s orbital
occupied by an unpaired valence electron from each hydrogen and an sp2 orbital from a carbon. A single
bond is always a sigma bond:
single bond = one σ bond
The third valence p orbital on each
carbon that was not involved in hybridization will be perpendicular to the
plane of the three sp2 orbitals. Side-to-side overlap between the parallel p orbitals, each
of which is occupied by carbon’s fourth unpaired valence electron that was not
used in sigma bonding, results in a second type of bond between the carbons
known as a pi (π) bond:
Therefore, a double bond is
comprised of one sigma and one pi bond:
double bond = one σ bond + one π bond
Consider the Lewis structure of C2H2:
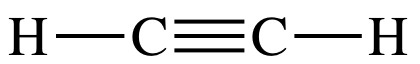
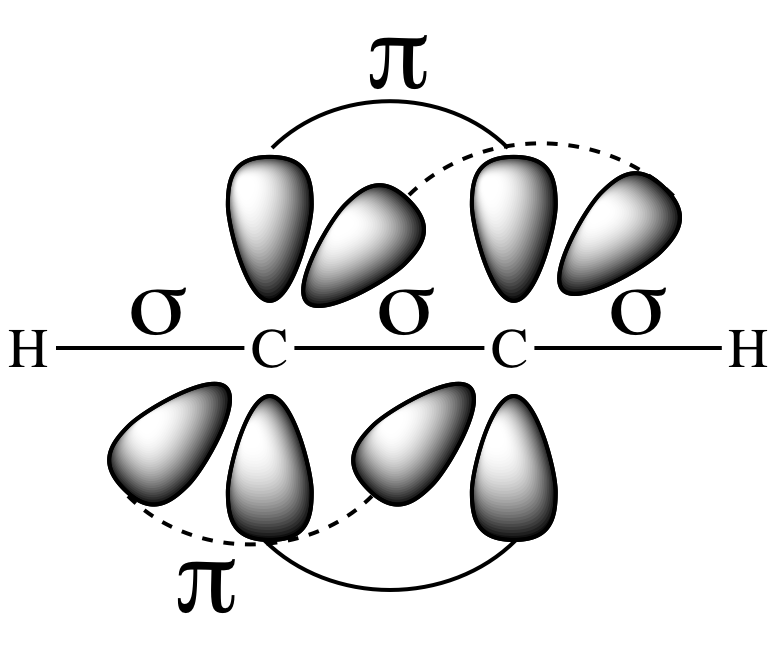
Each carbon atom in the molecule has
two sp hybrid orbitals (AB2), each of which is occupied by an
unpaired valence electron, that are 180° away from each other on opposite sides
of the carbon. A sigma bond will
be formed between the carbon atoms as a result of end-to-end overlap between
two sp orbitals, one from each carbon. In addition, sigma bonds will be formed between each
hydrogen and a carbon as a result of end-to-end overlap between the 1s orbital
occupied by an unpaired valence electron from each hydrogen and an sp orbital
from a carbon.
The other two valence p orbitals on
each carbon that were not involved in hybridization will be perpendicular to
each other and also to the line of the two sp orbitals. Side-to-side overlap between two pairs
of parallel p orbitals, each of which is occupied by one of carbon’s other
unpaired valence electrons that was not used in sigma bonding, results in two
pi bonds between the carbons:
Therefore, a triple bond is
comprised of one sigma and two pi bonds:
triple bond = one σ bond + two π bonds
Sample Exercise 11G:
Draw the Lewis structure for (NH2)2CO
that has no formal charges and determine the number of sigma and pi bonds in the molecule.
Solution:
2CO.png)
6 single + 1 double = 6 sigma + 1 (1 sigma + 1 pi) = 7 sigma bonds + 1 pi bond
Section 11-5: Molecular Orbital (MO) Theory
The Lewis structure shown below
suggests that O2 is diamagnetic because all electrons are paired:
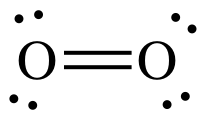
However, we know from experiment
that O2 is, in fact, paramagnetic due to unpaired electrons. Molecular orbital (MO) theory provides
an explanation for the paramagnetism of O2. MO theory suggests that the entire pool
of electrons in a molecule occupy a series of molecular orbitals rather than
being assigned to orbitals on individual atoms.
For homonuclear (same element)
diatomic molecules and ions from the second row of the periodic table, the
valence electrons will be assigned to molecular orbitals as follows:
A. When Li, Be, B, C, or N is
bonded, the order is: σ2s σ2s* π2p σ2p π2p* σ2p*
B. When O, F, or Ne is bonded, the
order is: σ2s σ2s* σ2p π2p π2p* σ2p*
Electrons that occupy bonding
molecular orbitals (no asterisk) will strenthen the bond between the atoms,
whereas electrons that occupy antibonding molecular orbitals (*) will weaken
the bond between the atoms. Sigma
(σ) molecular
orbitals occur singly and can be occupied by a maximum of two electrons. Pi (π) molecular
orbitals occur in pairs, with each orbital occupied by a maximum of two
electrons. Therefore, each set of
two pi orbitals can be occupied by a maximum of four electrons.
When molecular orbital diagrams are
written, two electrons that occupy the same orbital must have opposite
spins. Two electrons in a set of
pi orbitals must occupy separate orbitals and also must have parallel
spins. We can see from the
molecular orbital diagram below that O2 (6 + 6 = 12 valence
electrons) will have two unpaired electrons and, therefore, be paramagnetic:
↑↓
|
↑↓
|
↑↓
|
↑↓
|
↑↓
|
↑
|
↑
|
|
σ2s
|
σ2s*
|
σ2p
|
π2p
|
π2p
|
π2p*
|
π2p*
|
σ2p*
|
Bond order in MO theory is
analagous to the number of covalent bonds in a Lewis structure. We can determine the bond order for O2 as follows:
bond order = 0.5(number of bonding electrons –
number of antibonding (*) electrons) = 0.5(8–4) = 2
A bond order can be a fraction, and
a higher bond order will result in a shorter bond length. For example, O2+ has one less antibonding π2p* electron
than O2, which gives O2+ a bond order of 0.5(8-3)
= 2.5. Since O2+ has a higher bond order than O2, the bond length of O2+ will be shorter than the bond length of O2.
Sample Exercise 11H:
(a) Write the molecular orbital
diagram for N2 and determine the bond order. Also state whether N2 is
diamagnetic or paramagnetic.
(b) Is the bond length of N2- shorter or longer than the bond length of N2? Explain.
Solution:
(a) Recall that the π2p orbitals are
filled before the σ2p orbital
when Li, Be, B, C, or N is bonded. An N2 molecule has 5 + 5 = 10 valence electrons:
↑↓
|
↑↓
|
↑↓
|
↑↓
|
↑↓
|
|
|
|
σ2s
|
σ2s*
|
π2p
|
π2p
|
σ2p
|
π2p*
|
π2p*
|
σ2p*
|
bond order
= 0.5(8-2) = 3
no unpaired
electrons = diamagnetic
(b) N2- has
one more antibonding π2p* electron than
N2, which gives N2- a bond order of 0.5(8-3) =
2.5. Since N2- has a lower bond order than N2, N2- has a
longer bond length than N2.
If the bond order is determined to
be zero, a molecule will not be stable. For example, Ne2 (8 + 8 = 16 valence electrons) will not be
stable:
↑↓
|
↑↓
|
↑↓
|
↑↓
|
↑↓
|
↑↓
|
↑↓
|
|
σ2s
|
σ2s*
|
σ2p
|
π2p
|
π2p
|
π2p*
|
π2p*
|
σ2p*
|
bond order
= 0.5(8-8) = 0
However, if the molecule is ionized, the resulting ion may be stable. For example, Ne2+ has one less antibonding σ2p* electron than Ne2, which gives Ne2+ a bond order of 0.5(8-7) = 0.5. Since the bond order is greater than zero, Ne2+ will be stable.
Chapter 11 Practice Exercises and Review Quizzes:
11-1) Draw the Lewis structure,
name the molecular geometry (shape), draw a three-dimensional sketch, and
indicate the bond angle for each of the following molecules and ions:
(a) PH3
(b) NH2-
(c) NO3-
(d) O3
(e) NH4+
(f) NO2+
Click for Solution
11-1)
(a)
Lewis structure:
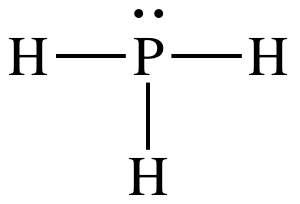
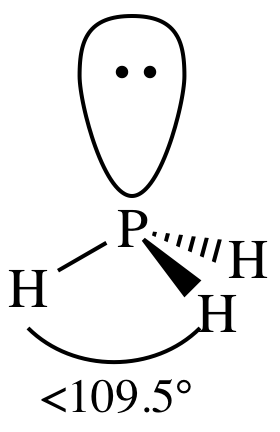
AB3E1
= trigonal pyramidal
3-D sketch:
(b)
Lewis structure:
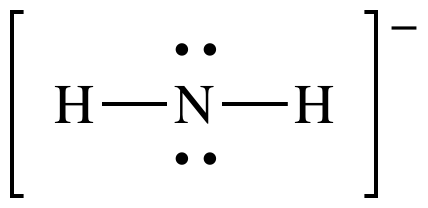
AB2E2
= bent
3-D sketch:
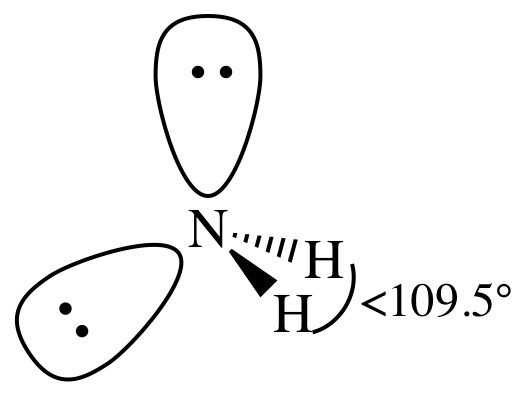
(c)
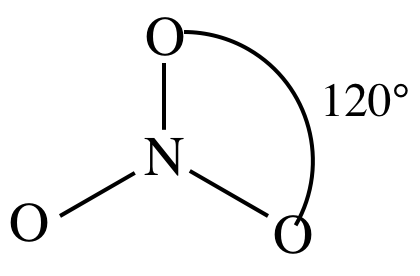
Lewis structure:
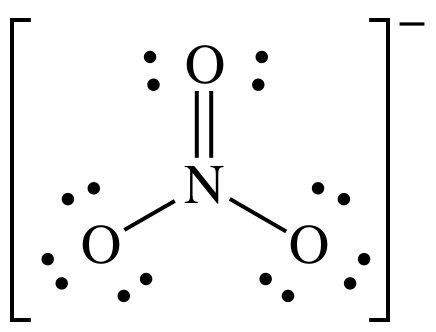
AB3
= trigonal planar
3-D sketch:
(d)
Lewis structure:
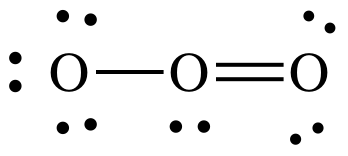
AB2E1
= bent
3-D sketch:
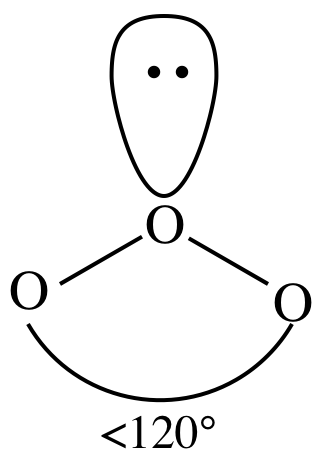
(e)
Lewis structure:
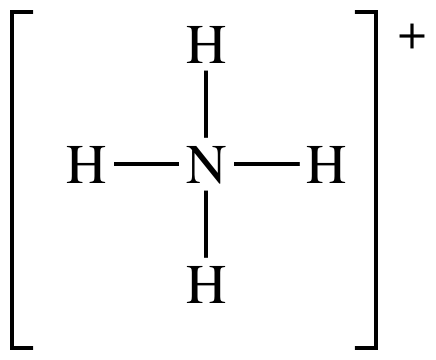
AB4
= tetrahedral
3-D sketch:
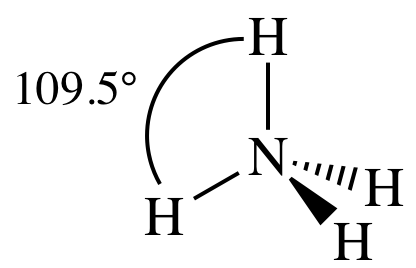
(f)
Lewis structure:
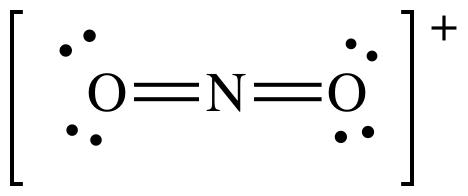
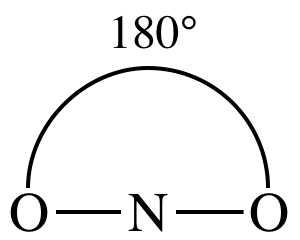
AB2
= linear
3-D sketch:
11-2) Draw the Lewis structure,
name the molecular geometry (shape), draw a three-dimensional sketch, indicate
the bond angle, and state whether each of the following molecules is polar or nonpolar:
(a) SiH4
(b) HOBr
(c) CS2
(d) NI3
(e) ClNO
(f) BF3
Click for Solution
11-2)
(a)
Lewis structure:
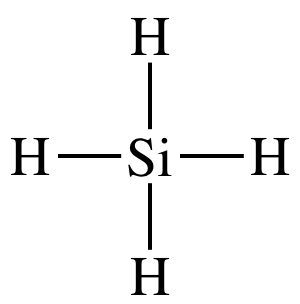
AB4
= tetrahedral
3-D sketch:
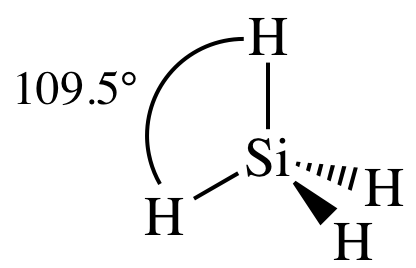
nonpolar molecule
(b)
Lewis structure:
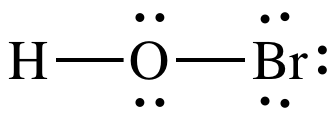
AB2E2
= bent
3-D sketch:
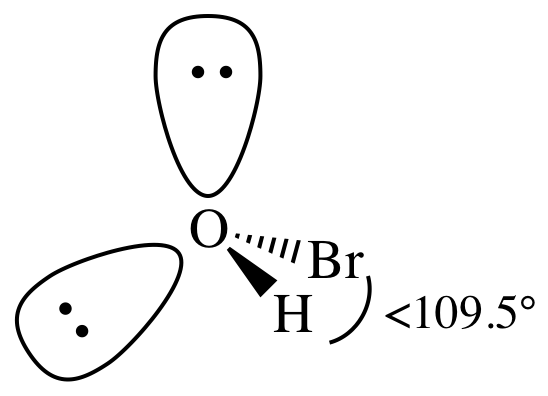
(c)
Lewis structure:
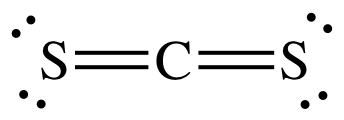
AB2
= linear
3-D
sketch:
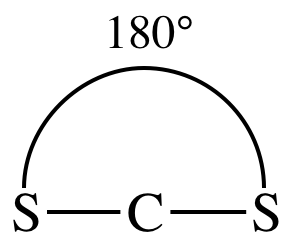
nonpolar molecule
(d)
Lewis structure:
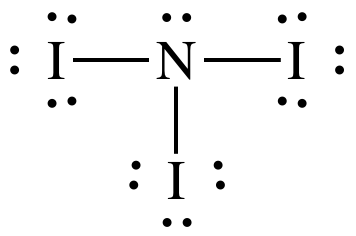
AB3E1
= trigonal pyramidal
3-D sketch:
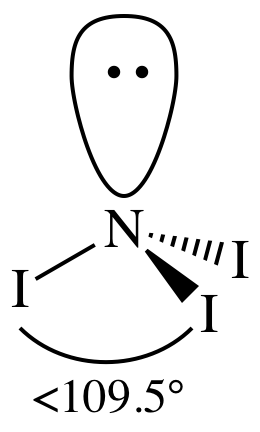
polar molecule
(e)
Lewis structure:
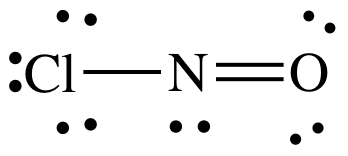
AB2E1
= bent
3-D sketch:
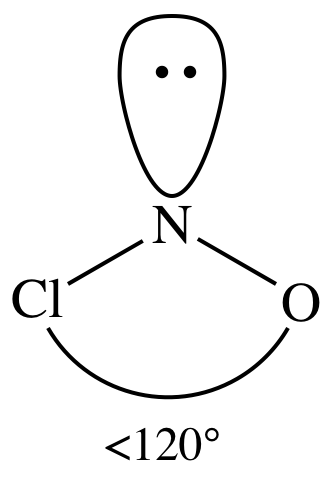
polar molecule
(f)
Lewis structure:
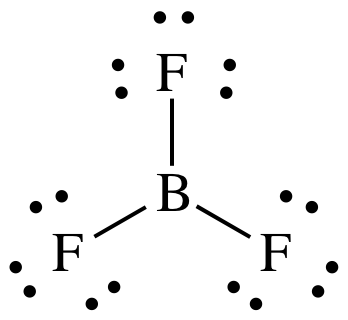
AB3
= trigonal planar
3-D sketch:
nonpolar molecule
11-3) Draw the Lewis structure,
name the molecular geometry (shape), draw a three-dimensional sketch, and
indicate the bond angle(s) for each of the following molecules and ions. Also state whether the neutral
molecules are polar or nonpolar.
(a) AsCl5
(b) IF3
(c) ICl6+
(d) RnF2
(e) SeCl4
(f) SF5-
(g) XeF4
Click for Solution
(a)
Lewis
structure:
AB5 =
trigonal bipyramidal, 3-D sketch:
nonpolar
molecule
(b)
Lewis
structure:
AB3E2 = T-shaped, 3-D sketch:

polar
molecule
(c)
Lewis
structure:
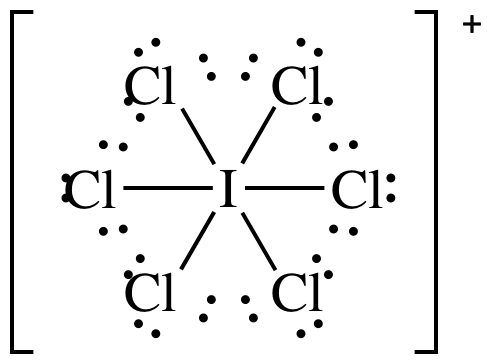
AB6 = octahedral, 3-D sketch:
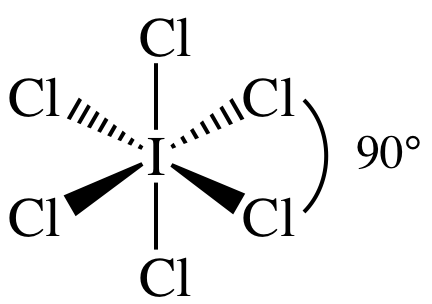
(d)
Lewis
structure:
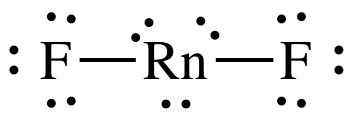
AB2E3 = linear, 3-D sketch:
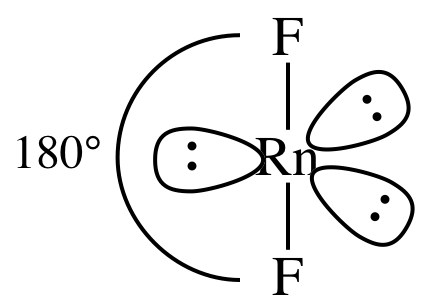
nonpolar
molecule
(e)
Lewis
structure:
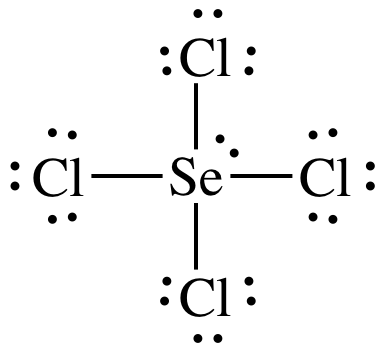
AB4E1 = seesaw, 3-D sketch:

polar
molecule
(f)
Lewis
structure:
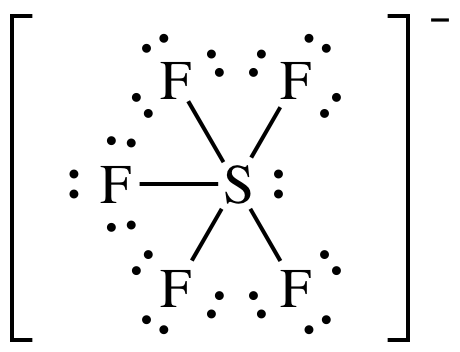
AB5E1 = square pyramidal, 3-D sketch:

(g)
Lewis
structure:
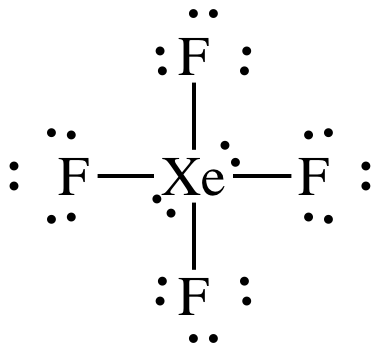
AB4E2 = square planar, 3-D sketch:
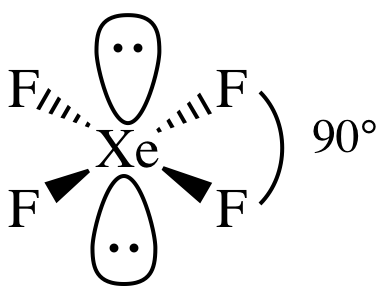
nonpolar
molecule
11-4) Draw the Lewis structure and
indicate the center atom hybridization for each of the following molecules and
ions:
(a) H2CO
(b) PH2-
(c) OCS
Click for Solution
(a)
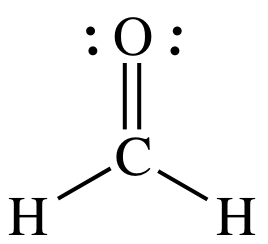
AB3 = sp2
(b)
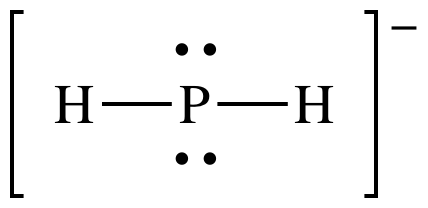
AB2E2 = sp3
(c)
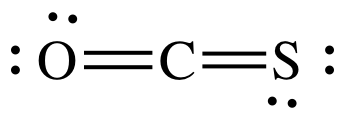
AB2 = sp
11-5) Draw the Lewis structure for CH3CN that has no formal charges and determine the number of sigma and pi bonds in the molecule.
Click for Solution
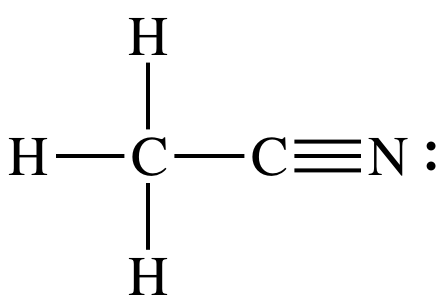
4 single +
1 triple = 4 sigma + 1(1 sigma + 2 pi) = 5
sigma bonds + 2 pi bonds
11-6)
(a) Write the molecular orbital
diagram for B2 and determine the bond order. Also state whether B2 is
diamagnetic or paramagnetic.
(b) Is the bond length of B2+ shorter or longer than the bond length of B2? Explain.
Click for Solution
↑↓
|
↑↓
|
↑
|
↑
|
|
|
|
|
σ2s
|
σ2s*
|
π2p
|
π2p
|
σ2p
|
π2p*
|
π2p*
|
σ2p*
|
bond order
= 0.5(4-2) = 1
has
unpaired electrons = paramagnetic
(b) B2+ has
one less bonding π2p electron than
B2, which gives B2+ a bond order of 0.5(3-2) =
0.5. Since B2+ has a lower bond order than B2, B2+ has a
longer bond length than B2.
