Chapter 21: Colligative Properties
Section 21-2: Freezing Point Depression, Boiling Point Elevation, and the van't Hoff Factor
Section 21-3: Experiment - Determining Molar Mass Using Freezing Point Depression
Section 21-4: Raoult's Law and Vapor Pressure
Chapter 21 Practice Exercises and Review Quizzes
Section 21-1: Molality
Molality (m) is another way to express the concentration of a solution and is
found by dividing the moles of solute by the kilograms of solvent:
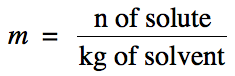
Sample Exercise 21A:
Calculate the molality of a
solution containing 17 grams of glucose, C6H12O6,
dissolved in 225 grams of water.
Solution:
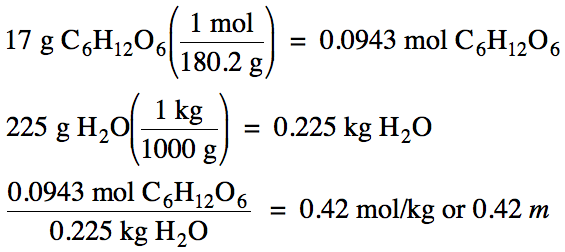
Molality in mol/kg can be used a
conversion factor between moles of solute and kilograms of solvent:
Sample Exercise 21B:
Calculate the mass of I2 that must be dissolved in 115 grams of carbon disulfide to create a 0.097 m solution.
Solution:
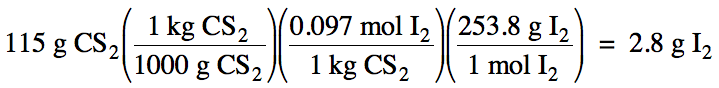
Sample Exercise 21C:
To create a 0.105 m solution, how many grams of
cyclohexane, C6H12, must be used to dissolve 12.6 grams
of C6H4Cl2?
Solution:
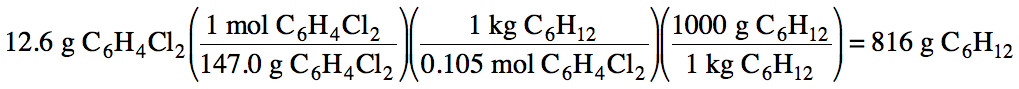
Section 21-2: Freezing Point Depression, Boiling Point Elevation, and the van't Hoff Factor
Colligative properties of
solutions, such as freezing point depression and boiling point elevation,
depend on the concentration of solute particles, but not on the identity of the
solute particles. When a solute is
dissolved in a solvent, the freezing point (tf) of the solution will
be lower than the freezing point of the pure solvent, and the boiling point (tb)
of the solution will be higher than the boiling point of the pure solvent.
The magnitude of the freezing point
depression (Δtf), or difference between the freezing points of the
pure solvent and the solution, can be calculated using the following equation
if the solute is molecular and does not ionize:
Δtf = Kfm
The magnitude of the boiling point
elevation (Δtb), or difference between the boiling points of the
solution and the pure solvent, can be calculated using the following equation
if the solute is molecular and does not ionize:
Δtb = Kbm
The freezing point depression
constant Kf and the boiling point elevation constant Kb (not to be confused with base ionization constant) have the unit °C/m and will be different for each
solvent. The freezing point
depression can be subtracted from the freezing point of the pure solvent to
find the freezing point of the solution. The boiling point elevation can be added to the boiling point of the
pure solvent to find the boiling point of the solution:
Sample Exercise 21D:
The freezing point of water is
0.0°C and the boiling point of water is 100.0°C. Given that Kf = 1.86°C/m and Kb = 0.52°C/m for water, calculate the freezing and boiling points of a solution containing
33 grams of sucrose, C12H22O11, dissolved in
46 grams of water.
Solution:
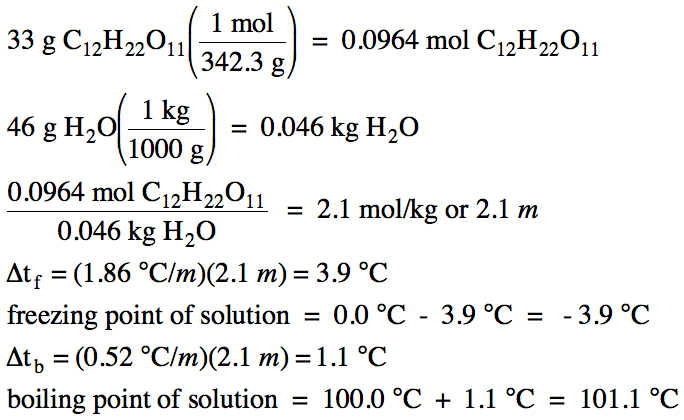
If the solute ionizes to some
extent during the dissolving process, the resulting concentration of solute
particles will be larger compared to a solution with the same molality in which
the solute is molecular and does not ionize. For solutions in which some or all of the solute is ionized,
the following equations can be used to calculate the freezing point depression
and boiling point elevation:
Δtf = iKfm Δtb = iKbm
The van’t Hoff factor i indicates the degree to which a solute
ionizes in water as follows:
Type of Compound
|
Value of i
|
molecular
(no ionization)
|
1
|
monoprotic
weak acid
|
1 < i < 2
|
monoprotic
strong acid
|
2
|
ionic
|
i = # ions in formula unit
|
Sample Exercise 21E:
Rank the following aqueous
solutions in order from lowest to highest freezing point and from lowest to
highest boiling point without performing detailed calculations:
0.010 m C6H12O6
0.010 m HF
0.010 m Na3PO4
0.011 m MgI2
0.015 m HCl
Solution:
Each solution has the same solvent
and, therefore, will have the same value of Kf in the equation Δtf = iKfm and the same value of Kb in the equation Δtb = iKbm. As such, a solution
with a larger product of (i x m) will have a larger freezing point
depression and a larger boiling point elevation. The solutions can be ranked from smallest Δtf and
Δtb at the top to largest Δtf and Δtb at the
bottom as follows:
0.010 m C6H12O6 = molecular (no ionization), i =
1: i x m = 1 x 0.010 m = 0.010 m
0.010 m HF = monoprotic weak acid, 1 < i < 2: 0.010 m < i x m < 0.020 m
0.015 m HCl = monoprotic strong acid, i = 2 (H+ + Cl-): i x m = 2 x 0.015 m = 0.030 m
0.011 m MgI2 = ionic, i = 3 (Mg2+ + 2 I-): i x m = 3 x 0.011 m = 0.033 m
0.010 m Na3PO4 = ionic, i = 4 (3 Na+ + PO43-): i x m = 4 x 0.010 m = 0.040 m
A solution with a larger Δtf will
have a lower freezing point. Therefore, the solutions will be ranked from lowest to highest freezing
point as follows:
0.010 m Na3PO4 < 0.011 m MgCl2 < 0.015 m HCl < 0.010 m HF < 0.010 m C6H12O6
A solution with a larger Δtb will have a higher boiling point. Therefore, the solutions will be ranked from lowest to highest boiling
point as follows:
0.010 m C6H12O6 < 0.010 m HF < 0.015 m HCl < 0.011 m MgI2 < 0.010 m Na3PO4
Section 21-3: Experiment - Determining Molar Mass Using Freezing Point Depression
The molar mass of a molecular
solute that does not ionize (i = 1)
can be determined using freezing point depression as follows:
1. Measure the mass of the solute.
2. Measure the mass and freezing
point of the pure solvent.
3. Dissolve the solute and measure
the freezing point of the resulting solution.
4. Given the Kf value
for the solvent expressed in the unit °C•kg/mol, solve for moles of solute in
the freezing point depression equation Δtf = Kf(nsolute/kg
solvent).
5. Divide the grams of solute by
the moles of solute to obtain the molar mass of the solute.
If we have determined the empirical
formula of the solute, we can use the molar mass we have calculated to
determine the molecular formula of the solute as follows:
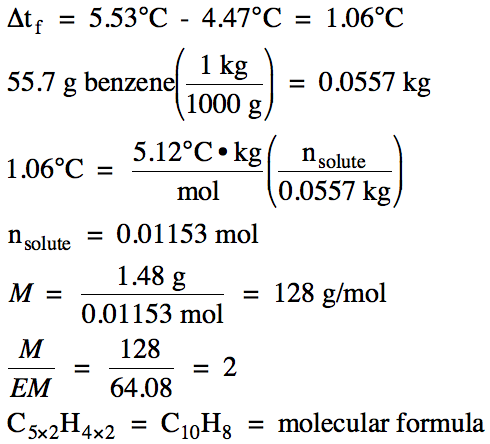
Sample Exercise 21F:
A molecular solute that does not
ionize has the empirical formula C5H4. A solution containing 1.48 grams of the
solute dissolved in 55.7 grams of benzene was found to freeze at 4.47°C. If Kf for benzene is 5.12°C/m and the freezing point of pure benzene
is 5.53°C, determine the molar mass and molecular formula of the solute.
Solution:
Section 21-4: Raoult’s Law and Vapor Pressure
The vapor pressure above a pure
liquid A can be denoted PA°. After a solute is dissolved in A, the vapor pressure of A above the
solution, PA, will be lower than PA°. If we focus only on solutes that are
molecular and do not ionize, the following version of Raoult’s Law can be used
to calculate the vapor pressure of A above a solution (where xA = mole fraction of A in
solution):
PA = xAPA°
= (nA/ntotal)PA°
If the solute is nonvolatile (has a
negligible vapor pressure and, therefore, does not add to the total vapor
pressure above the solution), then the total vapor pressure, Ptotal,
above the solution will be equal to PA calculated using Raoult’s
Law:
Sample
Exercise 21G:
The vapor pressure of pure water at
22°C is 19.8 mmHg. Calculate the
vapor pressure of water and the total vapor pressure above a solution
containing 205 grams of nonvolatile sucrose, C12H22O11,
dissolved in 575 grams of water at 22°C.
Solution:
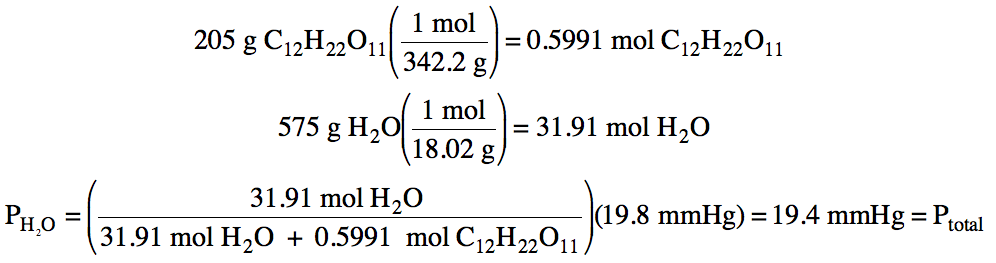
To determine the total vapor
pressure above a mixture of two volatile liquids A and B, Raoult’s Law must be
used twice to calculate the partial vapor pressures PA and PB above the mixture, after which PA and PB can be added to
obtain the total vapor pressure above the mixture. The mole fraction of A in the vapor, xA (g), will be equal to the ratio of the partial vapor
pressure of A to the total vapor pressure, as shown below:
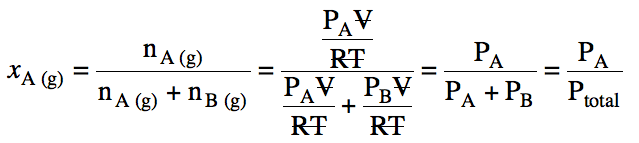
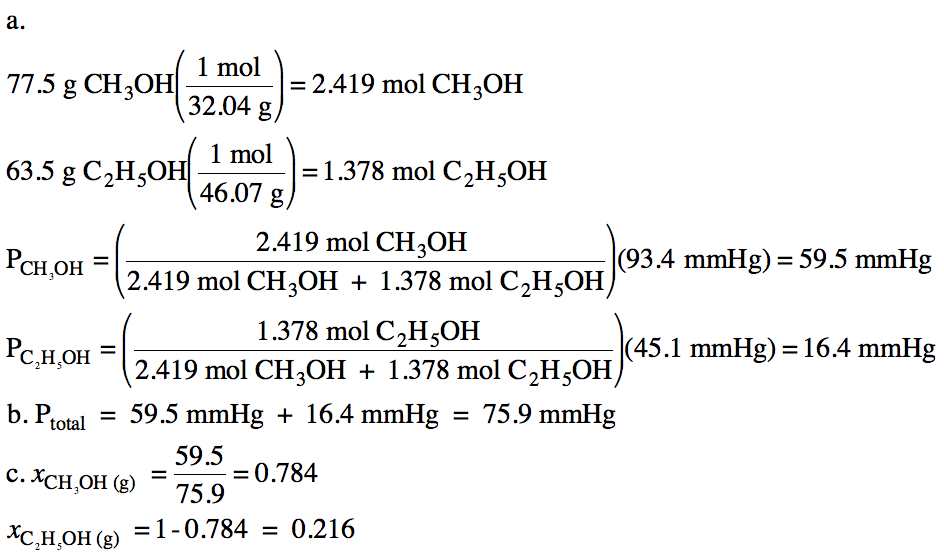
Sample Exercise 21H:
The vapor pressure of pure
methanol, CH3OH, at 21°C is 93.4 mmHg. The vapor pressure of pure ethanol, C2H5OH,
at 21°C is 45.1 mmHg. For a liquid
mixture containing 77.5 grams of methanol and 63.5 grams of ethanol at 21°C,
calculate:
a. The partial vapor pressures of
methanol and ethanol above the mixture.
b. The total vapor pressure above
the mixture.
c. The mole fractions of methanol
vapor and ethanol vapor above the mixture.
Solution:
Chapter 21 Practice Exercises and Review Quizzes:
21-1) Calculate the mass of naphthalene, C10H8, that must be dissolved in 175 grams of chloroform, CHCl3, to create a 1.2 m solution.
Click for Solution
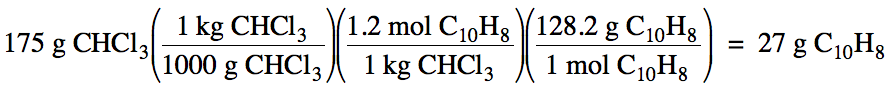
21-2) To create a 0.0855 m solution, how many grams of methanol
must be used to dissolve 0.548 grams of benzoic acid, C6H5COOH?
Click for Solution
21-2)
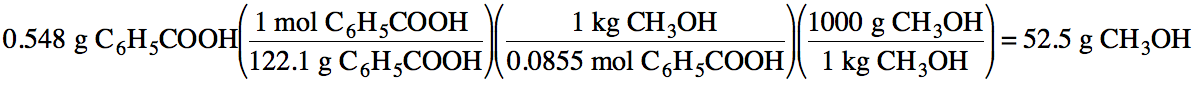
21-3) The freezing point of
cyclohexane, C6H12, is 6.6°C and the boiling point of
cyclohexane is 80.7°C. Given that
Kf = 20.0°C/m and Kb = 2.79°C/m for cyclohexane, calculate
the molality, the freezing point, and the boiling point of a solution
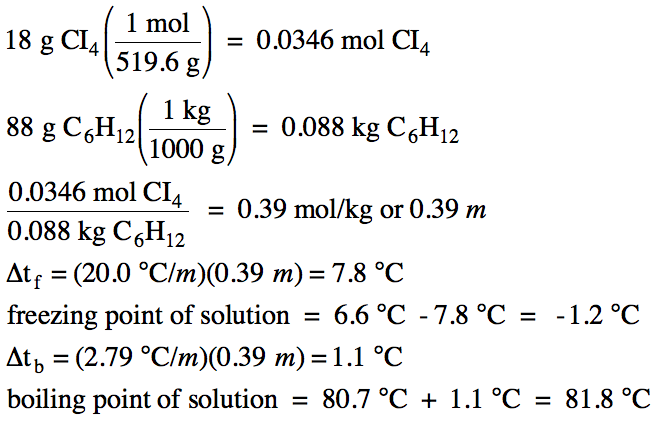
containing 18 grams of carbon tetraiodide dissolved in 88 grams of cyclohexane.
Click for Solution
21-3)
21-4) Rank the following aqueous
solutions in order from lowest to highest freezing point and from lowest to highest
boiling point without performing detailed calculations:
0.013 m CaCl2
0.014 m C12H22O11
0.014 m HC2H3O2
0.015 m KBr
0.017 m HNO3
Click for Solution
21-4) Each solution has the same
solvent and, therefore, will have the same value of Kf in the equation
Δtf = iKfm and the same value of Kb in
the equation Δtb = iKbm. As such, a solution with a larger product of (i x m) will have a larger
freezing point depression and a larger boiling point elevation. The solutions can be ranked from
smallest Δtf and Δtb at the top to largest Δtf and
Δtb at the bottom as follows:
0.014 m C12H22O11 = molecular (no ionization), i =
1: i x m = 1 x 0.014 m = 0.014 m
0.014 m HC2H3O2 = monoprotic weak acid, 1 < i <
2: 0.014 m < i x m < 0.028 m
0.015 m KBr = ionic, i = 2 (K+ + Br-): i x m = 2 x 0.015 m = 0.030 m
0.017 m HNO3 = monoprotic strong
acid, i = 2 (H+ + NO3-): i x m = 2 x 0.017 m = 0.034 m
0.013 m CaCl2 = ionic, i = 3 (Ca2+ + 2 Cl-): i x m = 3 x 0.013 m = 0.039 m
A solution with a larger Δtf will
have a lower freezing point. Therefore, the solutions will be ranked from lowest to highest freezing
point as follows:
0.013 m CaCl2 < 0.017 m HNO3 < 0.015 m KBr <
0.014 m HC2H3O2 < 0.014 m C12H22O11
A solution with a larger Δtb will have a higher boiling point. Therefore, the solutions will be ranked from lowest to highest boiling
point as follows:
0.014 m C12H22O11 < 0.014 m HC2H3O2 < 0.015 m KBr < 0.017 m HNO3 < 0.013 m CaCl2
21-5) (a) A molecular solute that
does not ionize was found to be 30.6% carbon and 1.7% hydrogen by mass, with
the remainder being bromine. Determine the empirical formula of the solute.
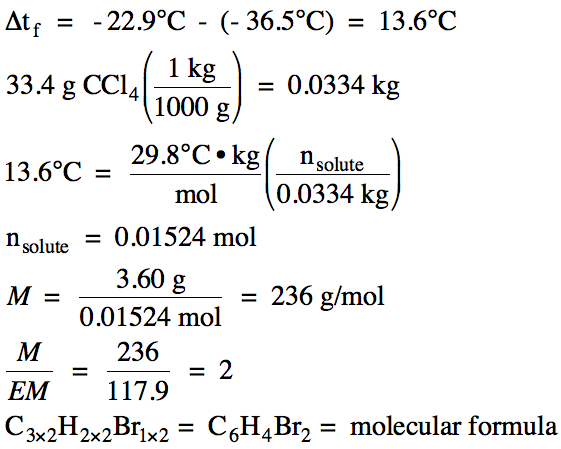
(b) A
solution containing 3.60 grams of the solute dissolved in 33.4 grams of carbon
tetrachloride was found to freeze at -36.5°C. If Kf for carbon tetrachloride is 29.8°C/m and the freezing point of pure carbon
tetrachloride is -22.9°C, determine the molar mass and molecular formula of the
solute.
Click for Solution
21-5) (a) 100% - 30.6% C –
1.7% H = 67.7% Br by mass
Assume one hundred grams of solute:
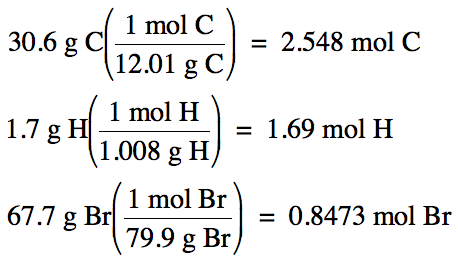
2.548 mol
C: 1.69 mol H: 0.8473 mol Br (divide each by 0.8473)
= 3 mol C:
2 mol H: 1 mol Br
C3H2Br
= empirical formula
(b)
21-6) The vapor pressure of pure
water at 35°C is 42.2 mmHg. Calculate the vapor pressure of water and the total vapor pressure above
a solution containing 10.0 grams of nonvolatile urea, (NH2)2CO,
dissolved in 175 grams of water at 35°C.
Click for Solution
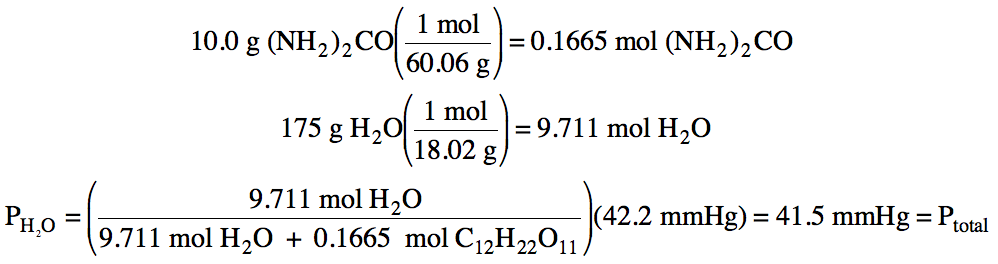
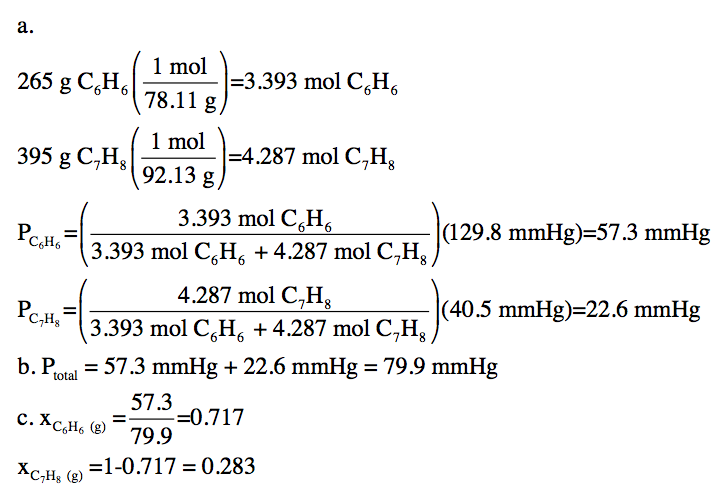
21-7) The vapor pressure of pure
benzene, C6H6, at 32°C is 129.8 mmHg. The vapor pressure of pure toluene, C7H8,
at 32°C is 40.5 mmHg. For a liquid
mixture containing 265 grams of benzene and 395 grams of toluene at 32°C,
calculate:
a. The partial vapor pressures of benzene
and toluene above the mixture.
b. The total vapor pressure above
the mixture.
c. The mole fractions of benzene vapor and toluene vapor above the mixture.
Click for Solution