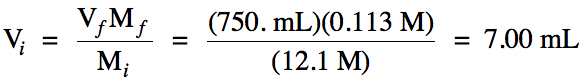Chapter 6: Solutions
Section 6-2: Solution Stoichiometry
Chapter 6 Practice Exercises and Review Quizzes
A solution is a mixture of one
substance, known as the solute,
dissolved in a second substance, known as the solvent. A common unit
used to express the concentration of a solution is molarity (M), which is found by
dividing the moles of solute by the total volume of the solution in liters:
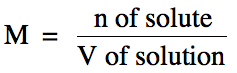
Note that we do not actually need
to know the volume or identity of the solvent to calculate molarity,
as demonstrated in the following problem:
Sample Exercise 6A:
Calculate the molarity
of a solution containing 7.6 grams of I2 dissolved to make 125 milliliters of solution.
Solution:
First, convert the mass of the
solute I2 to moles and then divide by the volume of the solution in
liters:
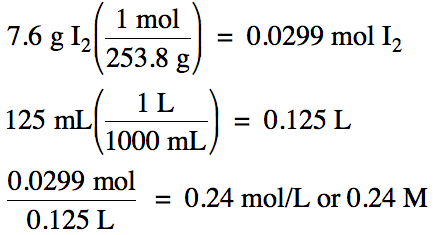
The molarity
equation above can be rearranged to solve for either moles of solute or volume
of solution:
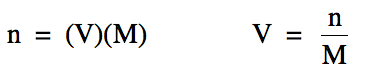
When finding moles of solute or
volume of solution, rather than plugging the measurements given in the problem
into one of the two equations above, we will instead use dimensional analysis
with molarity in mol/L as a conversion factor between
moles of solute and volume of solution as demonstrated in the following two
problems:
Sample Exercise 6B:
Calculate the mass of sucrose, C12H22O11,
dissolved in 500. mL of
0.140 M solution.
Solution:
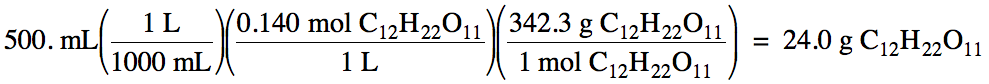
Sample Exercise 6C:
How many milliliters of 0.62 M
solution contain 3.18 grams of dissolved C10H8?
Solution:
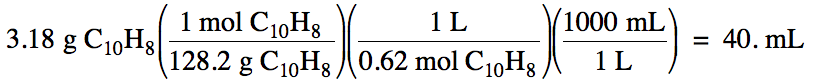
Section 6-2: Solution Stoichiometry
For reactions involving at least
one solution, we can use molarity to convert to
and/or from moles in stoichiometry problems, as
demonstrated below:
Sample Exercise 6D:
Given the unbalanced equation
below, if 15 mL of 0.46 M KOH react, what mass of
solid Mg(OH)2 will be produced?
Mg(NO3)2 (aq) +
KOH (aq) → Mg(OH)2
(s) + KNO3 (aq)
Solution:
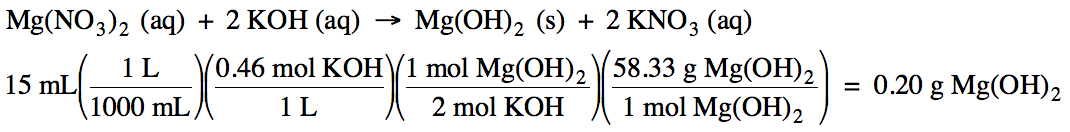
For reactions involving at least
one solution, molarity can also be used to convert to
and/or from moles in limiting reagent and percent yield problems.
Section 6-3: Dilution
When more solvent is added to
dilute a solution, the final volume increases and the final molarity
decreases, but the moles of solute do not change:
ni of solute
= nf
of solute
If we substitute the product of
volume and molarity for moles in the equation above,
we obtain an equation that is useful for many aspects of dilution (and also when solvent is removed through evaporation):
ViMi = VfMf
When using this dilution equation, note
that the volume must be in the same unit on both sides of the equation but does
not necessarily need to be changed to L and can remain in mL,
as demonstrated in the following problem:
Sample Exercise 6E:
A student is given a bottle from
the chemistry stockroom labeled 0.54 M NaCl. If the student removes 12 mL of solution from the bottle and adds water until a
volume of 36 mL is reached, what will be the new molarity of the solution?
Solution:
Solve the dilution equation for
final molarity:
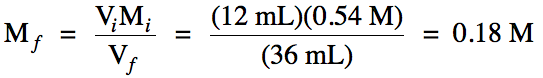
Chapter 6 Practice Exercises and Review Quizzes:
6-1) Calculate
the molarity of a solution containing 12.0 grams of C6H4Cl2
dissolved to make 975 milliliters of solution.
Click for Solution
6-1)
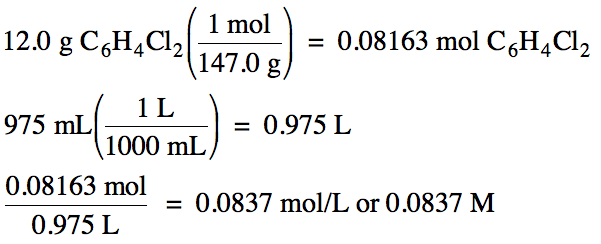
6-2) How
many milliliters of 0.40 M solution contain 0.575 grams of dissolved Br2?
Click for Solution
6-2)
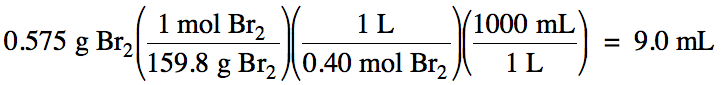
6-3) Given the unbalanced equation
below, how many milliliters of 0.334 M HCl are needed
to react with 3.82 grams of solid zinc?
Zn (s) + HCl (aq) → ZnCl2 (aq) + H2 (g)
Click for Solution
6-3)
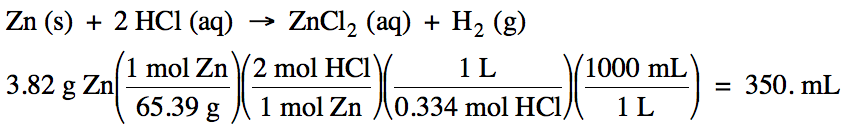
6-4) Given
the unbalanced equation below, if 4.58 grams of solid CaCO3 is mixed
with 75.0 mL of 0.645 M HI:
(a) Which is the limiting reagent?
(b) What is the maximum milliliters of carbon
dioxide gas that can form at 35°C and 712 mmHg?
(c) What mass of the excess reagent
remains when the reaction is complete?
CaCO3
(s) + HI (aq) → CaI2
(aq) + H2O (l) + CO2 (g)
Click for Solution
6-4)
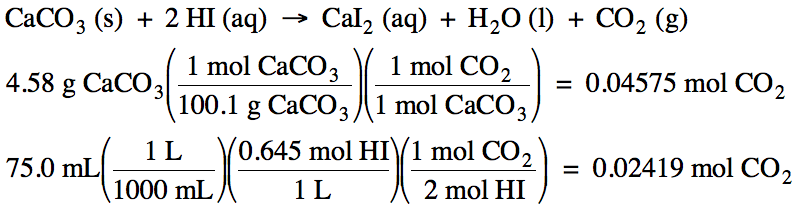
(a)
HI produces less carbon dioxide, so HI is the limiting reagent.
(b)
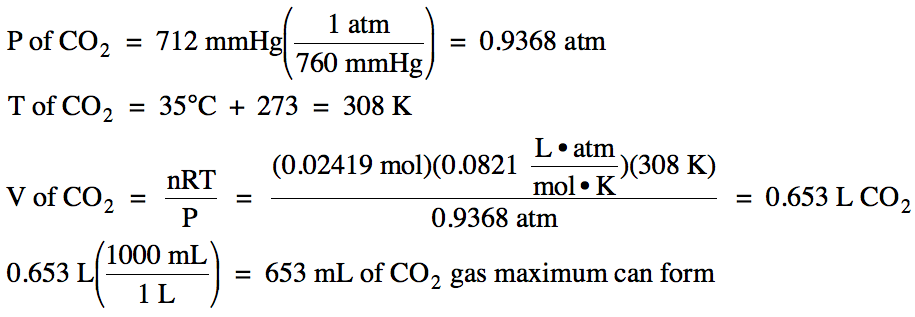
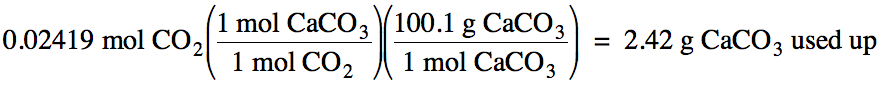
4.58 g – 2.42 g = 2.16
g CaCO3 excess
6-5) Given the unbalanced equation
below, if 85 mL of 0.162 M AgNO3 reacts
with an excess of Na2CO3 solution and then 1.8 grams of
Ag2CO3 is actually collected, what is the percent yield
of the reaction?
AgNO3
(aq) + Na2CO3 (aq) → Ag2CO3
(s) + NaNO3 (aq)
Click for Solution
6-5)
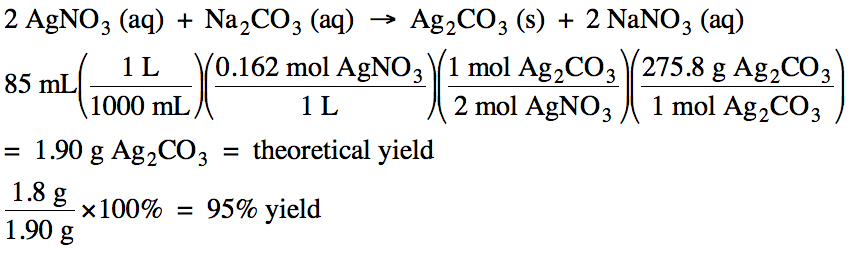
6-6) You
are in charge of providing 750. mL
of 0.113 M HCl for your class to use in an
experiment. If you are given a
bottle from the chemistry stockroom labeled 12.1 M HCl,
how many milliliters of this more concentrated solution must you take out of
the bottle and dilute with water in order to provide the correct volume and molarity of HCl solution for your
class?
Click for Solution
6-6)
Solve the dilution equation for initial volume: